Harnessing mass photometry for AAV sample characterization in GMP-regulated environments
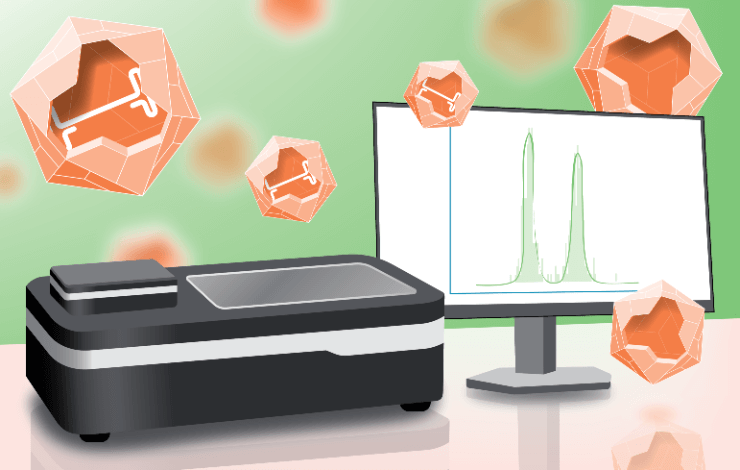
Mass photometry is a label-free bioanalytical technology that enables the characterization of AAV samples in solution by reliably measuring the empty-full AAV ratio using small sample amounts. This method can now be applied to GMP-regulated environments through new software solutions that are compliant with both FDA 21CFR11 and EU Annex 11.
In this webinar, we will explain how the new, compliant software solutions address all regulatory requirements, including user management, audit trails, and signing options when exporting files from Refeyn software applications. The new software solutions will be compatible with the SamuxMP and SamuxMP Auto mass photometers, which are optimized for AAV analytics. This allows the ability to rapidly measure AAV empty/full ratios with high precision and reproducibility for any AAV serotype.
Attend this webinar to enhance your understanding of:
- How to streamline AAV analytics in GMP environments
- How to enable the rapid, accurate, and reproducible analysis of full/empty capsid ratios across all AAV serotypes
- Software requirements to ensure regulatory compliance in both R&D and manufacturing
You have registered for this webinar
You might also like

Enabling early AAV characterization: the role of mass photometry in upstream process development

Assessing mRNA stability and integrity using mass photometry
Spot-on, standard-free AAV titer and characterization with Stunner
Advanced analytical strategies for AAV vector characterization using dPCR and long-read sequencing


