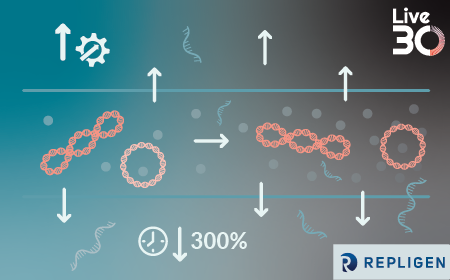
Discover how next-generation in-line analytical technologies and intelligent manufacturing strategies are redefining advanced therapy production. This session explores how Process Analytical Technologies (PAT) enable real-time process understanding and predictive control, laying the groundwork for truly continuous manufacturing of complex biologics including xRNA, mAbs, and viral products. Through practical data and case studies, speakers will reveal how real-time monitoring of raw material consumption, biomolecule generation, and byproduct accumulation is driving a fundamental shift toward faster, more consistent, and more cost-efficient manufacturing.
Attendees will gain a deeper understanding of how an integrated ecosystem approach can streamline process development, experimentation, and cGMP manufacturing. The session will also compare the performance and quality of continuous versus batch manufacturing as well as in-line versus offline testing, showcasing validated PAT applications in the continuous production of LNP-encapsulated mRNA.
The discussion will address strategies for implementing intelligent, next-generation manufacturing systems, highlighting how machine learning and predictive control can enhance speed, reduce costs, and improve product quality.
Attend for insights into:
- Real-world data on PAT implementation in continuous mRNA manufacturing and strategies for validation, compared against traditional QC testing
- How real-time analytics can track critical quality attributes (CQAs) for biomolecules, enabling faster decision-making and reduced production variability
- How an ecosystem-driven approach can unify data and control across bioprocess modalities to create stable, insight-led manufacturing Proven cost and time savings achieved through intelligent process control and machine learning integration
Secure your place now to discover how real-time quality insight and predictive control can redefine efficiency and reliability in advanced biomanufacturing.

Aaron Cowley
Chief Scientific Officer at Recipharm Advanced Bio
Dr. Aaron Cowley is the Chief Scientific Officer at Recipharm Advanced Bio, where he leads scientific strategy and innovation across the company’s advanced therapy platforms. With over 20 years of experience in biotechnology and pharmaceutical development, he is recognized for driving innovation through scientific rigor, technical excellence, and strategic leadership. Before joining Recipharm Advanced Bio, Dr. Cowley served as Chief Technical Officer and later CEO/President of Captozyme, where he guided the company’s growth and technological advancements for more than a decade. Earlier in his career, he held research and development positions at OxThera and served as a Postdoctoral Research Associate at the University of Georgia, gaining deep expertise in biochemical process development and preclinical program management. Dr. Cowley earned his PhD in Chemistry from the University of Kansas, an MBA from the University of Florida, and a BS in Biochemistry from Benedictine College. His combination of scientific insight and business acumen has made him a key force in advancing Recipharm’s mission to develop and deliver next-generation biotherapeutics.

Edita Botonjic-Sehic
Senior Director of Process Sciences at Recipharm Advanced Bio
Dr. Edita Botonjic-Sehic is Head of Process Analytics, Data Engineering, and Data Science at Recipharm Advanced Bio, where she leads the strategy and implementation of process analytical technology (PAT), data engineering, and data science to enable continuous bioprocessing. With over 20 years of experience, she has advanced the integration of analytical technologies and data-driven process control to enhance manufacturing efficiency and quality in pharmaceuticals and biologics development. Before joining Recipharm Advanced Bio, Dr. Botonjic-Sehic served as Director of Process Analytical Technology at Pall Life Sciences and Cytiva (Danaher companies), leading the development of novel analytical solutions. Her earlier career included leadership roles at GlaxoSmithKline, TEVA Pharmaceuticals, and Morpho Detection (formerly GE Homeland Security), where she managed government-funded programs focused on advanced detection and analytical systems. Dr. Botonjic-Sehic earned her PhD in Analytical Chemistry from the University of Rhode Island, dual undergraduate degrees in Chemistry and Mathematics from Assumption College, and a certificate in Business Analytics from Harvard Business School. She is a recognized thought leader, published author, and frequent speaker in the field of advanced process technologies.

Daniel Spurgin
Senior Director of Product Strategy and Marketing at Recipharm Advanced Bio
Daniel Spurgin is Senior Director of Product Strategy and Marketing at Recipharm Advanced Bio, a global CDMO specializing in advanced therapies, including mRNA and microbiome-based therapeutics and vaccines. In this role, he leads product strategy, marketing, and product initiatives supporting innovative approaches to continuous manufacturing and real-time process analytics. Daniel is part of the team that is launching Recimagine™ ecosystem—integrating process analytical technologies (PAT), simulated process development, and continuous processing capabilities to accelerate biotherapeutic development. With more than 15 years of experience in the biotechnology and bioprocessing industries, Daniel has held leadership roles in product management, marketing, and partnerships at Repligen and Phenomenex (a Danaher company). His work has contributed to multiple high-impact product launches and technology platforms supporting the manufacture of advanced biologics and gene therapies. Daniel holds a Master’s in Business and Science from the Keck Graduate Institute of Applied Life Sciences and a Bachelor of Science from Santa Clara University. He is passionate about advancing scalable, data-driven manufacturing solutions that improve the accessibility and quality of next-generation therapeutics.