Scalable production of AAV from shake flasks to 1,000 L single use bioreactors using the Gibco™ CTS™ AAV-MAX Production System
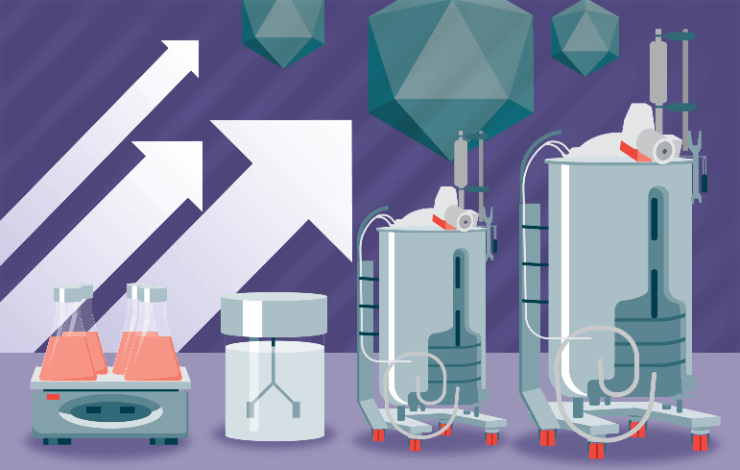
Production of high-titer AAV at clinical scale remains a significant challenge for the gene therapy industry. Developers need to understand how to optimize, scale, and qualify their manufacturing process for AAV production under the tightest of timelines. Due to high demand in the gene therapy space, effective solutions to address these challenges must be developed and implemented. Until recently, AAV production took place primarily in adherent cultures using 293T cells in the presence of fetal bovine serum; however, such adherent systems suffer from significant drawbacks including difficulty in scaling up, the presence of the SV40 large T antigen in the producer cell line as well as cost, consistency and regulatory considerations stemming from the use of animal sera.
To address these shortcomings, we present data on the Gibco™ CTS™ AAV-MAX Helper-Free AAV Production System, a chemically defined, suspension based AAV production system that allows for scalable, high-titer production of AAV viral vectors in a non-293T cell lineage. The AAV-MAX system comprises all of the components required for scalable AAV production in mammalian cells including: (1) a clonally-documented, high-titer 293F-derived producer cell line, (2) a chemically defined growth and expression medium, (3) a production enhancer, (4) a cationic lipid-based transfection reagent and booster, (5) a protein-free complexation buffer, and (6) a Polysorbate 20-based lysis buffer. Through a series of case studies, we demonstrate upstream and downstream approaches for consistent and scalable production, purification, and characterization of AAV at scales ranging from shake flasks to 50 L and 1,000 L single use bioreactors.
- How the AAV-MAX system can meet needs for consistent, scalable, and cost-effective AAV at all stages of development
- How to scale upstream AAV production to 1,000 L, including guidance and best practices for seed train, transfection, and other production parameters
- How to implement a downstream workflow for AAV purification at a 50 L scale
You have registered for this webinar
You might also like

AAV production and purification: key steps from design to GMP readiness
Industrialization of AAV manufacturing with Xcite® transient and stable production platforms

Platform-proof performance for AAV production in HEK293 cell lines

Scaling AAV5 production from bench to industrial scale: strategies for efficient, de-risked manufacturing