Feb
17
2022
On demand
AAV manufacturing process development using fast USP and DSP HPLC analytics
Thursday 08:00 PST / 11:00 EST / 16:00 GMT / 17:00 CET
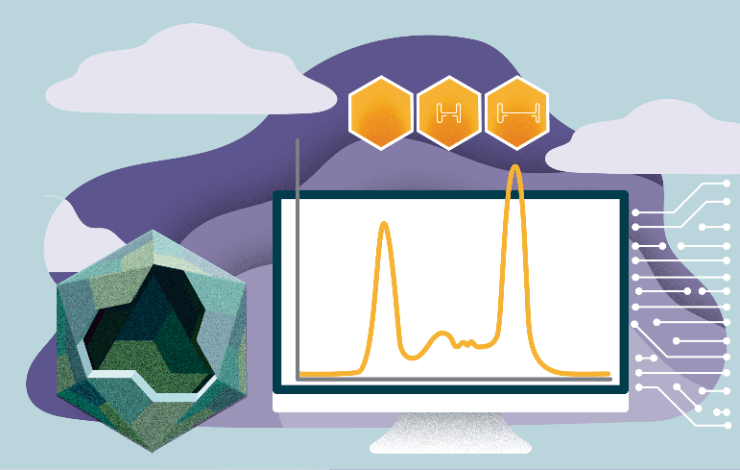
One of the key challenges in manufacturing viral vectors is to increase the ratio between empty and full capsids.
When the expression in the cell line results in less than 10% full capsid it is quite impossible to reach better than 90% full in the final product. It is therefore mandatory to optimise the USP to result in better empty/full ratio.
This can be efficiently realised by using at-line HPLC to allow for analysis of the full and empty capsids ratio directly in the harvest.
The residual empty capsids can be removed by polishing step using different anion exchange columns.
- AAV is the leading vector in the field of gene therapy and it is therefore crucial to develop a robust and high efficiency platform for its manufacturing.
- In-process analytics of vector capsid production is a critical optimization target in development of AAV-based gene therapy products.
- In this presentation we introduce a fast at-line HPLC based system that enables analysis of the full and empty capsids ratio directly in the harvest.
- Insights about purification will also be discussed.
You have registered for this webinar
You might also like

Strategies for streamlined manufacturing and analytics in CGT development
L Mueller, S Weber, D Windgassen
1 April
Watch

Accelerating AAV process development with high-throughput strategies
Dan Matuszek
28 October
Watch
Avoiding scale-up pitfalls: upstream process development strategies for AAV manufacturing
Sandy Tseng, Hugh Murray
26 February
Watch

Enabling early AAV characterization: the role of mass photometry in upstream process development
Maria Jacintha Victoria
24 June
Watch
Industrialization of AAV manufacturing with Xcite® transient and stable production platforms
Suparna Sanyal, Peng Wang
19 March
Watch

