cGMP manufacturing made easy: a case study by ElevateBio
Cell & Gene Therapy Insights 2021; 7(8), 1499–1512
10.18609/cgti.2021.198
The genetic revolution in medicine is set to fundamentally change the world over the next century. But while being at the forefront of developing advanced therapies is certainly exciting, it is not without challenges. Innovators are working to develop cell and gene therapies at a rapid pace – but it is critical that these products are developed not only quickly, but also correctly, the first time. In this article, we present a case study detailing ElevateBio’s work, in collaboration with Cytiva, to foster innovation and accelerate capabilities in cell and gene therapy through training, robust and reliable scale-up manufacturing, and capturing and leveraging manufacturing data.
Overcoming challenges & empowering innovation
Cell and gene therapy developers need access to expertise and talent, enabling technology, high quality process development, and good manufacturing practices (GMP) manufacturing – all of which present individual technical and executional risks. As a result, the cost of entry for cell and gene therapy development is very high. ElevateBio provides a solution to companies seeking to de-risk the development of innovative therapies through our technology, facilities and team, with technology enabled for protein, viral, and cell engineering, along with novel gene editing platforms.
ElevateBio was purpose-built for the development of technology to advance our own therapeutics as well as those of clients. An integrated approach is key – ideas are developed in the laboratory, and state-of-the-art processes are developed in the process development laboratory, translated internally, then manufactured in-house in GMP clean rooms.
We have two GMP facilities in one, where the manufacturing of viral vectors, gene therapy products and cell therapy products are segregated. The facility is designed to be able to support manufacturing of products that are manufactured for both US and EU clinical trials. This integrated approach to development, translation and manufacturing saves months in terms of development timelines as compared to a traditional outsourced model.
ElevateBio also possesses analytical development capabilities, so that we can rapidly develop and transfer methods, qualify them, and get ready for QC release testing, for the rapid disposition of products for these novel therapies.
In collaboration with Cytiva, ElevateBio has worked to address three areas that are crucial for the successful commercialization of cell and gene therapies:
- Team
- Training young professionals
- Process
- Ensuring reliable scale-up to cGMP
- Data
- System integration and analytics
Team: training tomorrow’s cell & gene therapy professionals
One challenge facing the industry is that experienced cell and gene therapy professionals are in short supply. ElevateBio works closely with university recruiting as well as internal teams to help train young professionals. We also use Cytiva’s Fast Trak™ Advanced CELLT1 course to enable accelerated team training, and allows us to hire and train young professionals and move them into GMP manufacturing quickly.
Along with our own experience, we use that course to accelerate the training of new process development teams, as well as manufacturing staff. The CELLT1 course combines both theory and laboratory work on end-to-end manufacturing in order to accelerate and standardize staff training.
Process: The FlexFactory™ platform
Part of the ElevateBio toolbox of enabling technologies is the FlexFactory platform from Cytiva, for both cell and gene therapy process development and manufacturing. Taking an integrated approach from early development through to clinical proof-of-concept, combined with the FlexFactory platform, helps to accelerate the development of both client and internal programs.
The FlexFactory platform includes Cytiva’s cell therapy instrumentation, qualification and documentation packages, project management consulting and training services, and the Chronicle™ automation software platform. It offers an integrated, scalable solution for acceleration and translation to GMP manufacturing, while reducing both cost of goods and risk (Figure 1
Viral vector manufacturing data
Cytiva technology was used in one of ElevateBio’s viral vector production suites, with the goal of rapidly building the infrastructure, team, and technology needed to support therapeutic development. The FlexFactory platform fit well with other scale-up solutions being used to develop the processes, and we were able to develop processes in the laboratory with confidence that they could be rapidly scaled and translated to GMP manufacturing, as demonstrated in Figure 2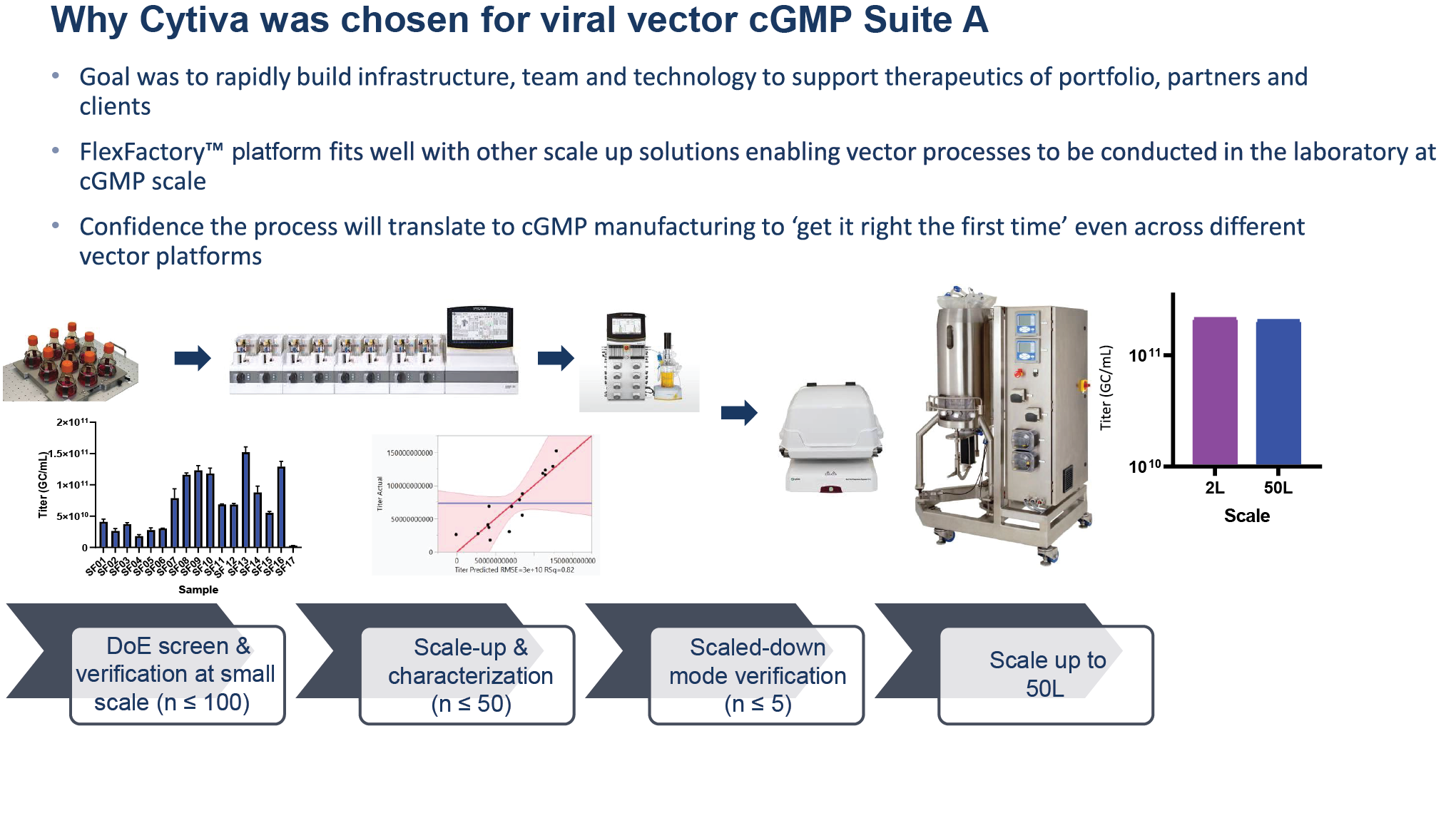
Figure 2 also shows an example workflow for adeno-associated virus (AAV), from a scale of 2 to 50 liters. Design of experiments (DoE), screens, and verification are performed at small scale, and may involve upwards of hundreds of conditions from a DoE perspective.
Next is scale-up and characterization. Once the scale-up and characterization are performed, justification of the scale-down model is completed through process development and protocols. The final conditions can then be optimized in the small setting before being translated and scaled up to 50 liters. This allows the process to move between 2 and 50 liters easily, with comparable results. The ability to screen multiple conditions at small scale and have confidence that the system will scale up effectively is crucial.
Figure 3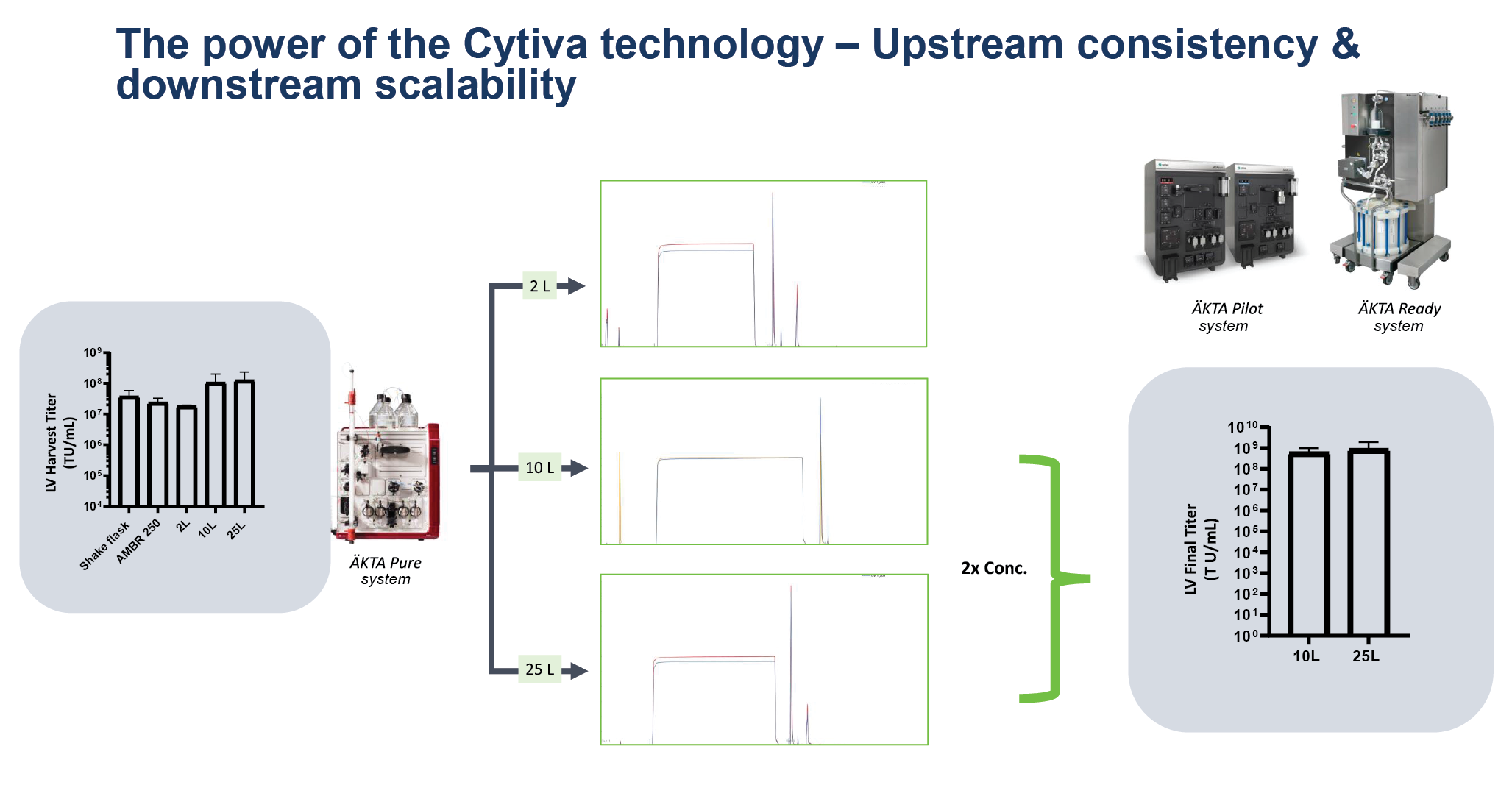
Data: capturing & leveraging data throughout the product lifecycle
ElevateBio is committed to building a highly digital infrastructure to capture and learn from data throughout the lifecycle of a product, and Cytiva’s solutions complement this robust manufacturing control strategy. This data can then be used for better process design through machine learning and artificial intelligence (AI).
Cytiva’s Chronicle automation software was integrated with our workflows (Figure 4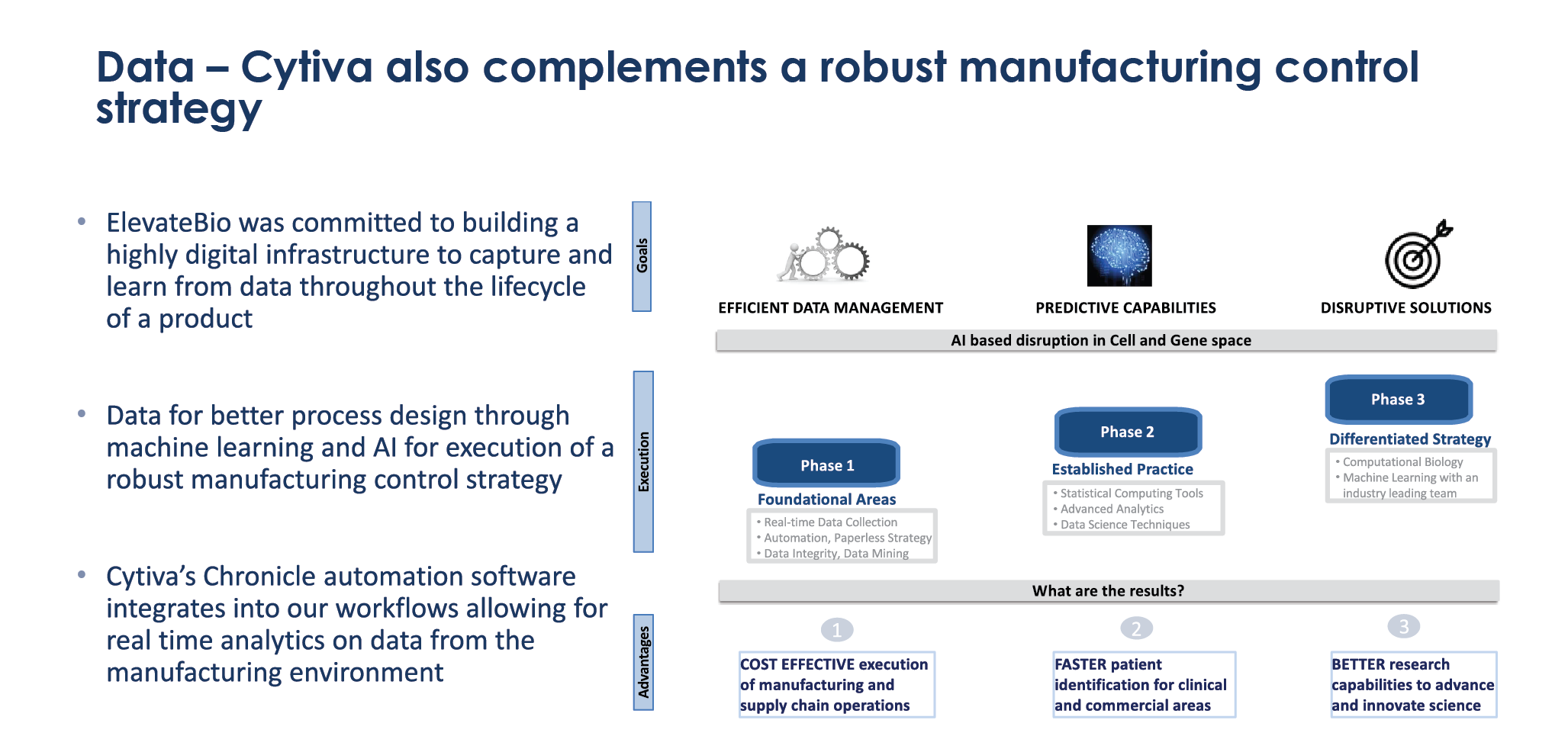
A fully integrated approach
Collaboration with Cytiva has helped ElevateBio meet the goals of establishing purpose-built technology and process automation that is adaptable to meet a diverse client and product portfolio. By taking a toolbox approach, it is possible to consider the intended product profile and use this to inform the process design – not only from an equipment perspective but also when considering raw materials, chemistry, manufacturing, and controls (CMC) and regulatory aspects, and clinical trials.
The FlexFactory platform enabled ElevateBio to build lentivirus and AAV platforms rapidly in the process development laboratory, and effectively scale them to GMP in the cleanroom. This is also applicable to cell therapy platforms, with a flexible process that allows the design and manufacture intent to be considered, whether for CAR T, TCR, or other cell therapy products.
ElevateBio also has in-house analytics, and can perform a high level of characterization for products. From an immunotherapy perspective we also have assays in house, both in vivo and in vitro, which can be correlated before bringing a product to clinical trial. The Chronicle data management system allows information from Cytiva equipment to be captured and integrated, so it can be used for machine learning approaches, resulting in further innovation and process improvement.
To summarize, in order to successfully accelerate cell and gene therapy development and increase the chance of ‘getting it right the first time’, developers should consider three key areas:
- Cell and gene therapy development is complex, so leverage collaborators with experience who can guide you to success with the end in mind;
- Cell and gene therapy talent is in short supply, and the environment is highly competitive. Train your existing team and standardize their manufacturing techniques;
- Consider collaborators who can provide access to next generation platform and manufacturing technologies, and a fully integrated end-to-end service model.
ASK THE EXPERTS
Q What are the main challenges you see for this industry?
SE: One is capacity – the shortage of places to do quality GMP work. The second is having trained staff to be able to do that work. A lot of companies such as Cytiva are working on training initiatives to be able to augment workforce development. In turn this will help support the growth in the contract development and manufacturing organization (CDMO) space.
The third is around automation and the removal of paper from the process; how much you can digitize your standard operating procedures (SOPs), batch records, and so on. It’s one of the things that keep popping up on US FDA and other regulatory writeups as issues in the space – how do we meet those challenges and get that early adoption, so that we can get past some of those hurdles around automation and digitization?
MP: I definitely agree. Having the knowledge of the development lifecycle – CMC regulatory experience and expertise especially – as it relates to novel therapies and GMP and raw material strategy are big challenges. Having that know-how, or access to it, is very important. In particular, having the documentation for rapid validation, and making sure systems are set up in a compliant way, is really important.
Q How does ElevateBio support the industry’s growth, and how can they help with some of these challenges?
MP: With the expansion that we have here at the facility, and the understanding of all the hurdles we have faced as we work with companies and build technology, we feel we can help companies and clients develop processes rapidly, but also give them options.
There is also a need for closing systems, or taking complex systems and simplifying them from a cost of goods perspective. What we can do is provide the knowledge and guidance to companies to help them develop that.
Our goal is democratization of technology and allowing folks to have access to it, whether it is companies coming and working with us, or allowing them to use these technologies and foster the development of their own clinical programs. I think that is why our model is different, and why we are very excited to help to enable the field in terms of development.
Q Shannon, can you tell us a little bit more about the FlexFactory platform, in terms of some of the benefits, and why was it suited to this project in particular?
SE: The benefits are getting to manufacturing as quickly as possible, and also establishing standardization around regulatory aspects and documentation – being able to get a qualified system, so you can move to production quicker.
We pride ourselves on documentation, whether we are supporting an internal regulatory group or a third party. Speed and quality are paramount. And a lot of that will tie in with work on next generation analytics. We have to consider how we can produce a product quickly, and how we tie in all the analytics, electronic SOPs, etc. And then, how can we have that framework already set up for ElevateBio and others, and have them hit the market as quickly as possible?
The other piece is that we are really in a shortage on training. Being able to train in parallel as you are developing and executing on FlexFactory installation and qualification is critical, and can save weeks or even months in training and validation.
MP: Regarding documentation and validation, building a facility at the same time as having access to that documentation rather than starting from scratch, was very important. We had to start from scratch for other systems, and that robust package and that documentation is not necessarily something that everyone offers you.
It is a big deal in terms of having something to access from early on to help guide you through the validation. Knowing in the background our systems and our equipment already had that ability and information was a big relief, and helped us to reduce staff as we built those systems.
In terms of working closely with the Cytiva team on the bioreactors and moving them in, we are very fortunate to have an amazing engineering staff and team that have a lot of know-how and speak the same language as the Cytiva team. Making sure that we had the level of rigor we needed from our facility, and knowing where we could go in terms of scalability and as we expand to multiple facilities, was important.
Regarding training, our curriculum is very much based on processes, but also common unit operations. That’s where can leverage Cytiva. We can send our curriculum to them, and they can train our staff on processes that are specific to our facilities and our programs. I think that’s also unique, and we appreciate the flexibility to provide that information to them and have them use that.
Q Shannon, can you speak to the importance of the digital automation and Chronicle software part of the workflow?
SE: I will continue the conversation around flexibility, because that is where a lot of it ends, if you will.
When we are looking to move towards digitalization of process analytical data, training is obviously important. Chronicle software is GAMP™ 5 certified, so it is going to provide that level of assurance that the process can be documented properly.
But training is a big portion of that, as well as flexibility. We talk about the flexible nature of putting in some of these processes, and having an automation and digitalization platform such as Chronicle software that can handle the flexibility. This means that quality groups can work with vendors such as Cytiva to have those standard platforms, standardization of the SOPs and electronic batch records and so on, but also be able to change them for each different product. Because every product requires a different process.
Having that flexibility where you don’t need someone that can write code is important. Chronicle software is web-based, and it is easy to make those changes for different products and different clients.
Q What were the main lessons learned from this project?
MP: For this project in particular it was really understanding what Cytiva can offer. When we built our automation team for example, understanding what the offering was and getting access to the team there was something that we probably should have taken even more advantage of at the beginning.
Go into a project with a really good user requirement and design intent, and work with the Cytiva team in making sure that you take the time to understand what the user requirements are.
As you build companies, as we all know, the opportunity is for you to hire experienced folks. But they come from different backgrounds, and as these projects evolve and you bring new programs and people on board, the user requirements may evolve and change. That happened with us and because we had documentation, we had procedures in place to be able to track those changes and remain flexible and evolve. I will admit I was probably the least experienced person as we deployed these types of automation strategies, but again, Cytiva worked closely with us and evolved with our team.
Another aspect is the long lead times. In the world of COVID, make sure you have a supply chain team that can keep an eye on the market and the environment – although a lot of this has of course been unexpected in the world, let alone in the biotech industry. Really keep an eye on the timelines and make an effort to get some of those long lead items ordered. Make sure that you have the ability to make those purchases early, so when you are ready to hand over the project and start those clinical batches, you are not delayed because of a long lead on a piece of equipment.
Cytiva is very good at keeping you informed about delivery times, construction times, even the supply chain. They worked really well with us, keeping us on track and on time for deployment, especially in our vector suite with the equipment and processes.
Q What advice would you give to other cell and gene therapy companies who are considering capacity expansion projects?
MP: The advice I would give is that oftentimes you have process development labs that may be working on different platforms, but you don’t necessarily have to scale and use the same platform in manufacturing.
I showed earlier that you can generate lots of data from a DoE perspective on different platforms. The Cytiva FlexFactory platform, especially in the production of viral vectors and manufacturing, works well with others from a decision perspective. So as we think about scaling and expanding capacity, we are very comfortable with our next scale-up and where we are going, and that it will be similar if not identical to where we are right now. Choosing those scale-down models, justifying those scale-down models, and getting to that next scale as quickly as you can to demonstrate that is important.
But again, don’t be afraid of understanding what you currently have in your facility, and be a little more open-minded on different solutions as you go into GMP and scale-up. This is something we learned early on. We did have some folks that were a little nervous about that – but the data speaks for itself, and we are very confident in what we built here.
SE: In this project with ElevateBio we were very lucky. Mike has built a top-notch team there that have a lot of years of experience under their belt in the cell and gene therapy space.
We talk about putting in a standard platform, but it’s not going to be standard for everybody. Developing flexibility around customers’ needs, especially a group like ElevateBio that has a lot of experience in the space, and providing them with platform documentation, regulatory support, and more, benefits us and makes us stronger.
Q What are some lessons learned you’d recommend to someone implementing a FlexFactory platform for the first time?
SE: What commonly comes up is flexibility and training. As groups are building their own infrastructure, they are hiring actively. Making sure that staff is up to date on the equipment and processes, especially in the pandemic world we live in, is difficult. Training in parallel as the FlexFactory platform is being deployed is critical.
Something that comes up a bit more often now, and which we’ve spent some time working on with ElevateBio, is around analytics. As every product is different, making sure the data from characterization, potency assays, and so on, can be integrated into a digitization platform is becoming more of a question that comes up. It is something I’d love to say we have figured out, but we haven’t completely yet. We are getting there, and our collaborations are really helping. ElevateBio’s analytic capabilities and know-how are very advanced, and we can benefit from that.
MP: The analytics are key. Our head of quality has built an amazing team, not just on qualification and validation and so on; the team here has an amazing analytical core. The amount of data we can generate, the samples we generate, are evolving the need for high throughput assays as well.
Analytics around not only the information we can get from the shop floor, but also on the tests at the end process, and the process analytics and technology associated with that, is something that we have the ability to offer to clients. Making sure you can look at the data as it comes off the shop floor rapidly to make those decisions and take it back to a design intent is really important.
Q From a manufacturing perspective, how does the FlexFactory platform address production risk management, versus other competing technologies that were considered when outfitting ElevateBio?
MP: Cytiva has done this for a long time. They have experienced shortages in the supply chain as they were integrating new technology, so they know about risk management.
For us, it was essentially day-to-day contact and conversation; a very active engagement. Cytiva saw where the industry was going, and helped us think about the supply chain risk. As soon as COVID started to really increase we put a team together to look at the entire supply chain at the facility from a risk perspective. Shannon and the team at Cytiva were very much integrated into that on a daily basis.
Other companies did this as well, but because that platform was so essential, and they understood that we had clinical demand coming, Cytiva worked really closely with us.
Q How does the FlexFactory quality system capture changes to systems that are already qualified under FlexFactory quality management system? How does your client know if the equipment needs to be re-qualified?
SE: For each piece of equipment you can put in when those need to be qualified and re-certified, depending on what is needed for each individual piece of equipment. Chronicle software can notify quality personnel, or whomever needs that information.
For the equipment that will be used you can barcode scan, QR scan, and make sure you are following a digital record that that piece of equipment is within certification. But we also do that with people. If someone is not trained on a certain process and tries to start a process, Chronicle software won’t allow it; it will stop and make sure the quality department is notified, so that the person can be properly trained, or another technician can come in and start initiating the process.
Q Regarding Cytiva’s quality documentation offering, how does the Cytiva quality management software product (QDMS) team implement continuous improvement? How are you able to keep up with rapidly changing technology and feedback from customers?
SE: We have a large qualification services group and quality group that maintain our documents such as: user requirement specifications (URS), or general specifications (GS) for each piece of equipment. If there is a change that needs to be made, commonly that is at a software level, what we call deviations. Those would run through our quality system, and the documentation would be changed within those parameters.
For each customer, we can again offer them the ability to be flexible and maybe alter the URS or GS depending on what the specific need is. Again, the equipment is platform equipment, individual unit operation, so we do that with not only Cytiva equipment but also third-party equipment. This is very important when we are providing a full solution for customers such as ElevateBio.
Being able to have that flexibility while also being able to start with a really good ground level understanding of the documentation that is required is something we pride ourselves on.
Q Does ElevateBio provide the people or consultants that help to decipher and analyze the data, or just the platform and tools to empower the customers to conduct their own analysis?
MP: The answer is a bit of both. We take a very embedded approach to our clients that are doing process development with us. Whether it is through what we have internally or what a client may have, we work very closely on sharing the data in the format they would most like it.
When someone works with us, it can be an idea, it doesn’t have to be a process that they have in hand. We sit down very closely with them and design the experiment and guide them through that development through our process development group.
They are welcome to come here to our facility and our BaseCamp. Project teams can sit down with our process development folks and get access to them, whether it’s through process design, how we create the process, or what they want from a clinical perspective. They can actually watch unit operations in the laboratories. Even if they don’t have that experience, we welcome them to join us on certain occasions at the facility.
Along with that of course comes the data – clients want data every day. We analyze it, and we interpret and talk about the data. Some like to take the data on their own, so put it into their own tools and electronic systems, and present it that way. I think folks really enjoy working with us because we take a lot of ownership in our process development programs and treat them as if they’re our own, because they are. Whether it’s a company that we are building on our own, or a client, they are all the same.
Whether it is through process design, all the way through data sharing, we are working on some really cool ways to provide almost real-time access. The only reason we can do that is because of the automation strategies that we have built and the equipment that we have – the infrastructure and the analytics.
Q Looking to the future, what’s the next series of projects you are planning at ElevateBio?
MP: Expansion of capabilities. We have projects like our inducible pluripotent stem cell lines helping to evolve regenerative medicine products. The gene editing, Life Edit technology and building therapeutics around that, and getting folks to have access to those enzymes, is very exciting for us. We are building the processes for those types of products, and again we have that experience internally.
We are planning the expansion of all those capabilities, but also the expansion of our process development. What we’re seeing is there’s a lot of excitement in the industry for working with ElevateBio to start early, develop a process, and then do GMP manufacturing. But also, because we have a GMP manufacturing capacity available right now, there are folks that may already have a process that they will want to transfer in and have us run.
There are a multitude of ways in which we’re expanding, and the next series of projects involve working with more and more clients and biotechnology companies of various sizes to use our capacity and our team to help develop these novel therapies.
The expansion of the Cytiva platform continues as well, and this is just the first step of our viral vector capabilities. We know we would like to collaborate with Cytiva, because they evaluate technology that is out there from a process perspective, an automation perspective, and scale that. We want to be part of that as well.
So a lot of projects, and we are actively expanding all of that infrastructure right now so we can continue to work with more and more clients and technology companies, which is really exciting.
Authorship & Conflict of Interest
Contributions: All named authors take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Acknowledgements: None.
Disclosure and potential conflicts of interest: Dr Eaker is a full-time employee of Cytiva. The authors have no other conflicts of interest.
Funding declaration: The authors received no financial support for the research, authorship and/or publication of this article.
Article & copyright information
Copyright: Published by Cell and Gene Therapy Insights under Creative Commons License Deed CC BY NC ND 4.0 which allows anyone to copy, distribute, and transmit the article provided it is properly attributed in the manner specified below. No commercial use without permission.
Attribution: Copyright © 2021 Cytiva. Published by Cell and Gene Therapy Insights under Creative Commons License Deed CC BY NC ND 4.0.
Article source: This article is a transcript of a previously published webinar, which can be found here.
Webinar published: Oct 14 2021; Publication date: Nov 24 2021.