
Welcome to the Analytics Hub!
Explore a curated collection of technical insights, strategic guidance, and practical advice on leveraging the latest analytical tools to enhance the identification and measurement of critical quality attributes.
Designed as the go-to resource for professionals working to identify, characterize, and quantify unwanted contaminants- helping you stay ahead with next-generation tools built to deliver the speed, sensitivity, and reliability the industry demands.
Register once to access a piece of content and the whole collection will be unlocked with topics including:
 Analytical development
Analytical development Cell therapy manufacturing
Cell therapy manufacturing Expression systems
Expression systems Mycoplasma testing
Mycoplasma testing Regulatory CMC
Regulatory CMC Residual DNA testing
Residual DNA testing Vector production
Vector production
Any Questions?
Content

Residual DNA testing: enhancing efficiency and regulatory compliance
Shriya Sahu
7 October 2025
Watch

How do I get started with analytical assays? Top tips
26 September 2025
Poster
Optimizing for faster quality control in cell therapies: leveraging rapid detection methods
Srinath Kashi Ranganath
8 September 2025
Poster

Unlock advanced strategies in cell therapy analytics
S Peterson, K Shum, A Chandran et al.
4 September 2025
Watch

A practical guide to fit-for-purpose analytical development in an emerging cell therapy landscape
Damian Marshall, Jie Wei, Florian Durst
18 June 2025
Expert Roundtable
Optimizing for faster quality control in cell therapies: leveraging rapid detection methods
Srinath Kashi Ranganath
5 June 2025
Watch

How do I ensure my early-stage analytical development is ‘phase-appropriate’? Top 5 tips
3 June 2025
Infographic
Fit-for-purpose analytical development for an emerging cell therapy landscape
J Wei, D Marshall, F Durst
3 April 2025
Watch

eBook: Increasing speed and efficiency in biotherapeutics analysis
12 February 2025
EBook

Early-stage analytical development strategies for cell therapy
Ramon Mendoza, Kyle Carter, Seth Peterson
16 January 2025
Expert Roundtable

Balancing precision and efficiency in cell therapy assays: low volume sampling for mycoplasma detection
Sharon Rouw
10 December 2024
FastFacts

Preparing for success in gene therapy analytical development
Yan Zhi, Jing Li, Andres Castillo
2 December 2024
Expert Roundtable

Cell therapy analytics: INFOGRAPHIC
22 November 2024
Infographic
Preparing for success in gene therapy analytical development
Y Zhi, J Li, A Castillo
9 October 2024
Watch

Early-stage analytical development strategies for cell therapy
R Mendoza, K Carter, D Kamikura et al.
26 September 2024
Watch
4 key lessons learnt from choosing in-house assay development
17 September 2024
Infographic
Leveraging dPCR for residual DNA and viral titer quantitation in advanced therapy manufacture
Maya Dubey
14 August 2024
Watch
Analytical strategies for sterility and mycoplasma testing in biotherapies: from early development to production scale-up
Sharon Rouw, Michael Brewer
4 April 2024
Watch

5 dos and don'ts for regulatory compliance in AAV manufacturing
4 April 2024
Poster
Overcoming challenges in cell therapy production: rapid sterility testing
Seth Peterson
13 March 2024
Watch

What goes into developing an in-house method for quantitation of residual host cell DNA?
Ilaria Scarfone
12 March 2024
Webinar Digest

Regulatory considerations and validation strategies for mycoplasma testing for cell-based therapies
Michael Brewer
9 February 2024
Webinar Digest

Addressing regulatory guidance for HEK293 cells and AAV-based therapeutics manufacturing
Michael Brewer
15 December 2023
Webinar Digest
Simplifying residual DNA quantitation and biotherapeutic manufacturing
Ilaria Scarfone
13 December 2023
Innovator Insight

Streamlining nucleic acid extraction for biotherapy manufacturing: manual and automated solutions for success
Suzy Brown
30 November 2023
FastFacts

Considerations for affinity capture in an AAV platform downstream process
Jett Appel
22 November 2023
Innovator Insight
Simplifying residual DNA quantitation in biotherapeutic manufacturing
Ilaria Scarfone
19 October 2023
Watch

Analyzing lentivirus particles using dPCR techniques
Samyuktha Shankar
21 September 2023
FastFacts

Considerations for affinity capture in an AAV platform downstream process
Jett Appel
20 September 2023
Watch

Improving biopharmaceutical quality and safety by implementing a microbial identification strategy in the workflow
Nico Chow
14 September 2023
Webinar Digest
Regulatory considerations & validation strategies for mycoplasma testing for cell-based therapies
Michael Brewer
14 September 2023
Innovator Insight

Navigating evolving regulatory CMC guidance in the AAV gene therapy field
M Brewer, A Cockroft, C Fuentes et al.
24 August 2023
Poster

Addressing regulatory guidance for HEK293 cells & AAV-based therapeutics manufacturing
Michael Brewer
23 August 2023
Innovator Insight
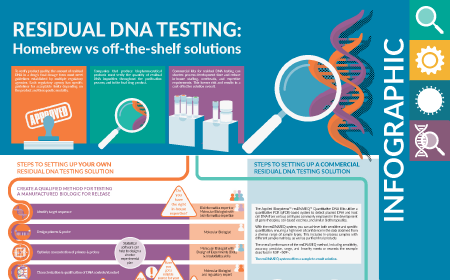
Residual DNA testing: Homebrew vs off-the-shelf solutions: INFOGRAPHIC
17 August 2023
Infographic
Regulatory considerations and validation strategies for mycoplasma testing for cell-based therapies
Michael Brewer
20 July 2023
Watch
Addressing regulatory guidance for HEK293 cells and AAV-based therapeutic manufacturing
Michael Brewer
29 June 2023
Watch
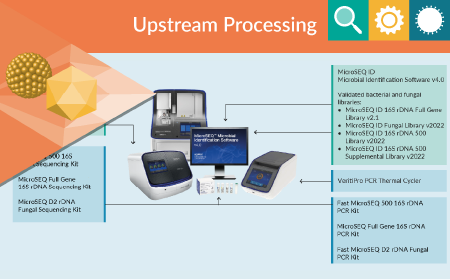
Environmental monitoring: optimizing microbial control in cell & gene therapy workflows
Nico Chow
20 June 2023
Innovator Insight
Optimizing the cell therapy patient journey through integrated CRO/CDMO partnership
Panteli Theocharous, Rupa Pike
6 June 2023
Watch

Navigating evolving regulatory CMC guidance in the AAV gene therapy field
M Brewer, A Cockroft, C Fuentes et al.
15 May 2023
Expert Roundtable

Simple analytical tools for detection of impurities in biologics produced using the Sf-baculovirus platform
Maya Yovcheva
1 May 2023
FastFacts
Environmental monitoring: optimizing microbial control in cell and gene therapy workflows
Nico Chow
27 April 2023
Watch

Navigating evolving regulatory CMC guidance in the AAV gene therapy field
Y Zhi, C Le Bec, M Brewer et al.
22 March 2023
Watch

Analytical innovation: meeting the demands of commercial viral vector manufacture
16 March 2023
EBook

Lentiviral titer determination: rapid & robust molecular methods suitable for validation
Unnati Dev
10 March 2023
Webinar Digest
Lentiviral titer determination: rapid & robust molecular methods suitable for validation
Unnati Dev
23 February 2023
Innovator Insight

Mycoplasma testing: regulatory guidance and strategies for cGMP cell and gene therapy manufacturing
Mike Brewer
15 December 2022
Webinar Digest

Robust quantitation of residual host cell and plasmid DNA & oncogenic fragments in HEK-based viral vector manufacturing
16 October 2022
FastFacts
Simplifying analytical development of viral vector production: robust and sensitive methods for common expression systems
Srinath Kashi Ranganath
16 July 2022
Innovator Insight

Removing technological barriers to efficient large-scale LV vector production
S Jeffers, E Jackson-Holmes, R Lopez de Maturana et al.
27 June 2022
Innovator Insight

Removing technological barriers to efficient large-scale LV vector production
R Lopez de Maturana, E Jackson-Holmes, M Neri et al.
26 April 2022
Watch
Simplifying analytical development for viral vector production: robust and sensitive methods for common expression systems
Srinath Kashi Ranganath
3 March 2022
Watch
Key factors to consider for successful cell therapy manufacturing: a case study
2 March 2022
Podcast
Residual DNA testing in viral vector manufacture: exploring the challenges and solutions
Ilaria Scarfone, Mike Brewer
22 February 2022
Podcast

Expression systems for viral vector production: advantages of the Sf9 baculovirus system and simple solutions to address its specific analytical challenges
Yi Fang Lee, Srinath Kashi Ranganath
24 October 2021
Innovator Insight
Manufacturing and analytics for lentivirus and AAV vectors: a visual and audio guide
20 August 2021
Infographic

Development & validation of a robust commercial solution for measuring residual kanamycin-resistant plasmid DNA
Tania Chakrabarty
9 August 2021
FastFacts
Expression systems for viral vector production: advantages of the Sf9 Baculovirus system and simple solutions to address its specific analytical challenges
Yi Fang Lee, Srinath Kashi Ranganath
22 July 2021
Watch

Mycoplasma detection in cell therapy products: GMP-compliant implementation & validation of a commercial real-time PCR assay for routine quality control & lot release
Valentina Becherucci
20 June 2021
FastFacts

Viral vector production process intensification: analytics, automation, in-line testing and more
9 February 2021
Expert Roundtable

Viral vector production process intensification: analytics, automation, in-line testing, and more
S D’Costa, M Delahaye, M DiBiasio-White et al.
18 January 2021
Expert Roundtable Video
Scalable AAV manufacturing – Addressing challenges across the workflow
K Thompson, C Yan Liu, J Li et al.
29 October 2020
Watch

Combining state-of-the-art production, purification and analytics to optimize AAV manufacturing for clinical and commercial gene therapies
B Pence, R Snyder, K Torchilin
10 December 2019
Watch

AAV vector process development: achieving high purity and high yield – experiences from the frontline
M Bakhshayeshi, M Mercaldi, M Hebben et al.
6 June 2019
Expert Roundtable

AAV vector process development: achieving high purity & high yield – experiences from the frontline
M Bakhshayeshi, M Mercaldi, M Hebben et al.
4 June 2019
Expert Roundtable Video