Big pharma case study: alpha testing of a generic anti-AAV kit and comparison to in-house methods
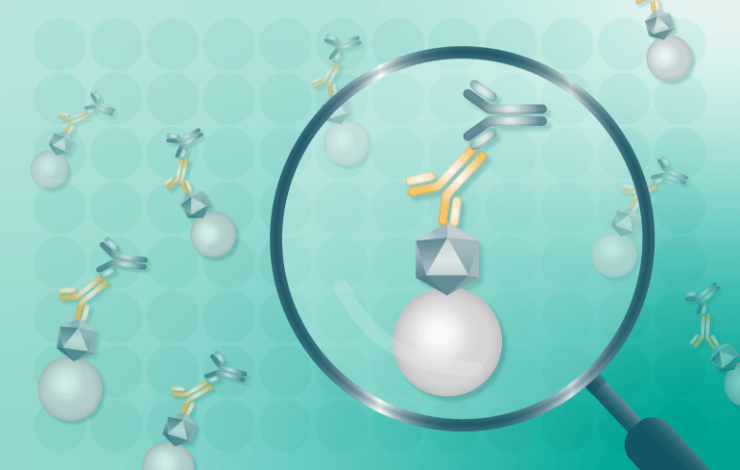
Gene delivery via AAV vectors has emerged as a pivotal technology in the clinical treatment of human diseases. The non-pathogenic viruses transduce cells, leading to a long-term expression of clinically relevant transgenes. However, due to the high prevalence of anti-AAV antibodies from prior exposure to the non-pathogenic wildtype AAV, which can diminish the effectiveness of the therapy, testing is necessary to assess levels of pre-existing AAV immunogenicity antibodies for individual candidates included in clinical trials.
This webinar will present a case study from Bayer comparing a commercially, generic anti-AAV assay run on an automatic immunoassay platform designed to detect anti-AAV antibodies in human and cynomolgus monkey matrices with results obtained from in-house validated ELISA plate assays. Both human and monkey serum samples were tested using the generic anti-AAV assay to determine the levels of pre-existing AAV-antibodies for 2 different AAV serotypes. By utilizing cut-point validation, individual samples were ranked as anti-AAV antibody positive or negative in both assays and the results were directly compared.?The speakers will compare the ranking profiles obtained from the two alternative assays to identify the optimal tool for investigating levels of pre-existing AAV-8 and AAV-9 antibodies in human and monkey matrices.
Attend this webinar to:
- Learn how to optimize pre-existing anti-AAV antibody analysis of candidates for nonclinical and clinical studies
- Assess comparative case studies around detection of anti-AAV8 and -AAV9 antibodies in human and monkey serum samples
- Compare and contrast the performance of traditional ELISA plate-based assays with an automatic immunoassay platform approach in anti-AAV antibody quantification across multiple serotypes
You have registered for this webinar
You might also like
A case study in faster, higher-quality pDNA manufacturing

Case study: establishing a versatile and intensified lentiviral vector production platform with TFDF
Conducting rapid microbiological monitoring in fill-finish with biofluorescent particle counters: a collaborative case study
Scaling smarter: a case study in overcoming cell therapy manufacturing and regulatory hurdles



