End-to-end automated manufacturing of low-seed CAR-T cells
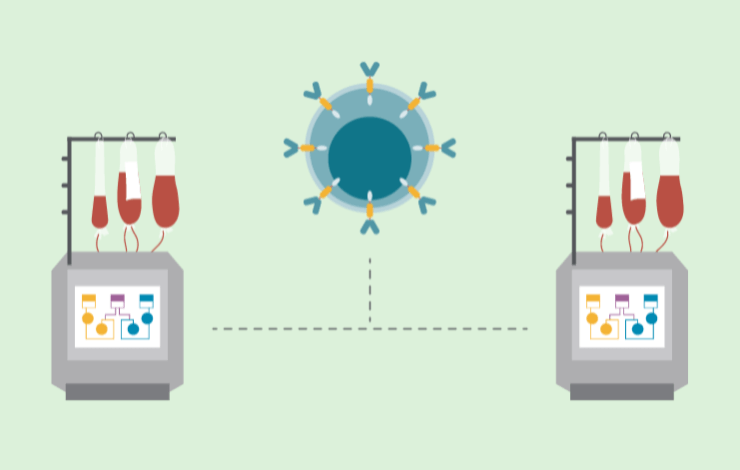
The pathway to commercial success requires viewing commercial readiness through several lenses, including scalability, process development and optimization, cost and labor management, and GMP compliance.
For example, in CAR-T cell production, the upstream process must allow for a broad dynamic range in terms of cell seeding density - for example, to cater for both pediatric and adult dosages. A detailed investigation into the expansion of genetically modified CAR-T cells on the Quantum Flex Cell Expansion System was recently completed as part of a strategic collaboration between BioCentriq and Terumo Blood and Cell Technologies. This presentation not only details the technical results of this study, but also the rapid technology transfer of an end-to-end manufacturing protocol to a contracted site.
Join this webinar to learn more about:
- Data describing cell expansion kinetics of CAR-T cells and deep phenotypic characterization
- Insights into the critical factors involved in the rapid technology transfer of an end-to-end manufacturing protocol to a contracted site
- Results of cell killing assays and cytokine re-stimulation responses
You might also like
Key considerations for shortening your CAR-T manufacturing process

Armor CAR T cells with enhanced potency for cancer immunotherapy
Closed and automated CAR-T production: a fast, simple workflow for high-dose, high-quality cells
Commercialization of CAR-T therapy in New Zealand: clinical insights & scalable manufacturing