Expand your T cell manufacturing capacities in an automated, closed system to shorten your production time
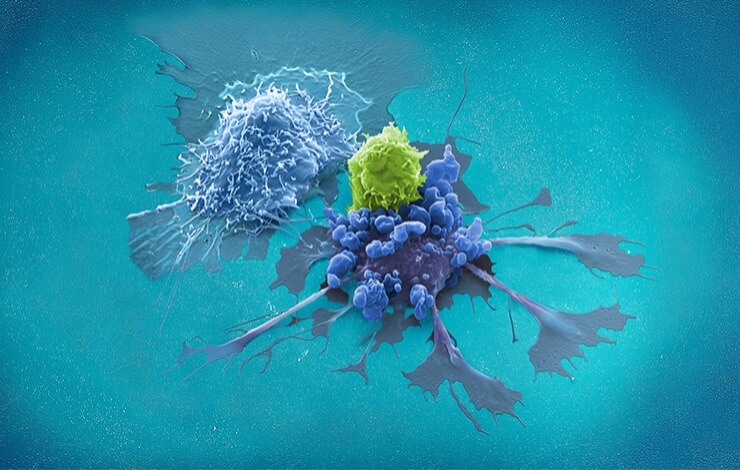
The recent successes of chimeric antigen receptor (CAR) T cell therapy in hematologic malignancies have led to tremendous interest in the immunotherapeutic field, and the potential of genetically modified T cells now expands into solid tumors and infectious diseases. However, current manufacturing processes for CAR T cells are complex and labor-intensive.
In this two-part webinar you will learn more about the new T Cell Transduction – Large Scale (TCT-LS) process for the CliniMACS Prodigy® Platform. We share process details and differentiators, along with limitations and how to choose the right process type for your requirements. Furthermore, we will walk you through recent data on TCR-modified T cell manufacturing for AML treatment, and how the overall process was shortened to only 8 days.
Join this webinar to gain insights into:
- The benefits of manufacturing within an automated and functionally closed system
- How to manufacture large amounts of genetically modified T cells
- How large culture capacities give the option to reduce manufacturing time
You have registered for this webinar
You might also like
Closed and automated CAR-T production: a fast, simple workflow for high-dose, high-quality cells
Sharpen up your RNA and LNP quant with Stunner
Collaboration in action: decentralized manufacturing to expand patient access in cell therapy

Flow with confidence: innovations that foster flexibility and scale in closed-system manufacturing



