How short-term gain can lead to long-term pain
Cell Gene Therapy Insights 2017; 3(4), 271-284.
10.18609/cgti.2017.018
INNOVATOR INSIGHT
For the commercial success of advanced therapies including CAR-T, they must not only be safe and effective, but also consistently produced and delivered to patients at a cost substantially less than the reimbursable rate. Regardless of whether your therapy is allogeneic or autologous, initial protocols are usually developed using laboratory equipment and skilled research staff completing aseptic operations in BSCs. What many advanced therapy companies either fail to realize or choose to ignore is that the decisions made during early development have a profound and long-lasting impact on the success of the therapy. This article provides valuable insight into some of the factors critical to successful commercialization of an advanced therapy to help prevent short-term gains from becoming long-term pain.
Accelerating commercialization
The CAR-T market is highly competitive with several therapies in development that are achieving very impressive patient responses. Hence for CAR-T therapy companies, time to market has become one of the most critical factors for commercial success and one that potential investors are acutely aware of.
Changes to the regulatory environment are also requiring a more pro-active strategy for scale-up. Take Japan, for example, where conditional market approval can now be achieved after completing a relatively small Phase 1/2 trial. The implications of Japan’s accelerated approval process are not yet fully understood. However, one consequence is the need to invest in facilities and equipment capable of commercial scale Good Manufacturing Practice (GMP) manufacturing much earlier than companies would typically consider necessary or face the brutal reality of commercializing an expensive manual process prone to error.
One of the most significant time-to-market risk factors is the possibility of being required to repeat clinical trials because of:
- Inconclusive clinical data;
- Late changes to the manufacturing process that challenge product comparability and hence the data on which prior trials and approval has been based.
Implementing an appropriate closed and automated process during early clinical trials can substantially prevent this from occurring, whereby:
- Closing the process reduces the risk of contamination during processing and enables a significant reduction in the cost of building and operating clean room manufacturing facilities;
- Automation substantially reduces the number of operators and level of skill required and ensures that the process is reproducible.
Don’t leave (too much) value on the table
Whether your company’s objective is an initial public offering (IPO) to commercialize the therapy yourself or an early exit via acquisition by a major market player, it is important not to leave too much value on the table.
I have spent considerable time in mature manufacturing environments including Big Pharma, where the cost of goods (COGs) is crucial to long-term commercial success and in some cases, survival. I have also experienced the frustration of not being able to make relatively simple design changes to a regulated product, despite the significant cost benefit of doing so. Perhaps it is difficult for those that have not experienced this first hand to truly appreciate how important it is to minimize the COGs during development. Perhaps there are so many things that are critical now that future problems necessarily get less attention.
Ultimately the price you can get for your therapy will be largely out of your hands, being defined by market forces such as reimbursement relative to the efficacy and cost of existing products, and the need to be affordable for patients and clinicians. The price will also likely need to reduce with time as commercial pressures come to bear and competitor products enter the market.
The gross margin of your product is what is left after paying for the COGs and needs to cover your indirect costs including sales and marketing, HR, R&D, distributors, etc. plus provide sufficient profit to keep your shareholders happy. Consequently, regardless of what your long-term strategy is, COGs will be a vital factor in determining the current value of your business.
To put this ‘value’ into perspective, we have modeled the impact of investing in closed automated processing to reduce the COGs for a CAR-T therapy by $5000 per patient. Assuming a gradual ramp up to treating 10,000 patients per year you would generate a staggering $400 million additional profit within the first 10 years of commercial production (Figure 1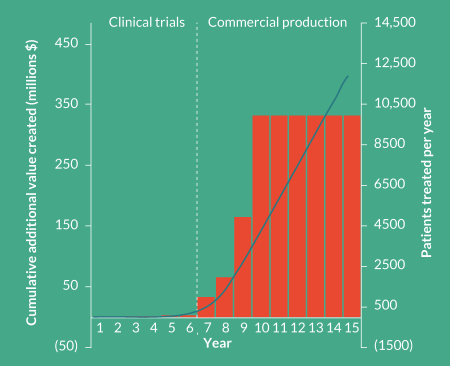
I often hear the argument from companies that they understand the benefit of automation but simply cannot afford the additional capital investment required at this stage in their development. Perhaps surprisingly, in most cases the payback would actually be achieved prior to commercial production through savings in labor and capital amortization alone. Unfortunately, when this ‘value’ becomes obvious or critical to the business, it may be too late or much more difficult to realize.
Your process and the ultimate COGs are locked in much earlier than you realize
Like it or not, your process and the COGs is substantially defined in your Investigational New Drug (IND) application. “You should include all of the components and materials used during the manufacture of the gene therapy product, such as the vector, cells, cell bank systems and any reagents or excipients. In addition, you should describe all procedures used during the manufacturing process.” [1]
This also applies to the Quality Assurance/Quality Control (QA/QC) and batch release tests that you carry out on your product.
Although it is expected that your process will evolve throughout development, any change from what is defined in the IND will require some level of comparability assessment, especially as you progress into the later phases of your clinical trials.
Figure 2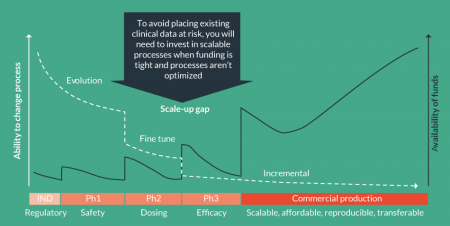
For development of advanced therapies, the IND describes the manufacturing process and the associated reagents. Both are fundamental to defining your product.
Coming to terms with the ultimate COGs for a specific therapy is a critical and complex process with many factors that need to be considered. We have been involved in several such activities for a range of advanced therapies throughout the world. Based on this knowledge we created a representative CAR-T process and cost model assuming scale-up of a typical manual process using open or semi-closed processes in class B processing suites. This model was then used to explore various manufacturing scenarios. In Figure 3 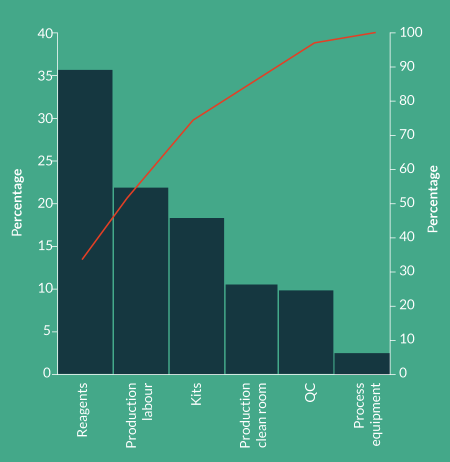
Reagents
Reagents represent >35% of the COGs for the protocol modeled. It is very important to note that the model was based on a single cell selection step using magnetic beads at a cost of $2500 per patient dose. For many of the therapies being developed, the volume and type of beads being used could add as much as $10,000 to the COGs. In fact it is not uncommon to see reagents representing 70% of the COGs. Since reagents are fundamental to defining the product, making changes to reagents during or after clinical trials would represent a significant comparability risk. With many reagents only able to be sourced from a single supplier, negotiating substantial price reductions, even at scale, is likely to be difficult.Choose reagents wisely and optimize your process design to reduce the volume of reagents consumed.
Labor
Assuming average US labor rates for appropriately qualified and trained staff, labor is the next major contributor to COGs at 22% and correlates directly with the number of production personnel required. What isn’t captured in the model are the significant indirect costs associated with hiring, training and managing hundreds of highly qualified staff performing complex and repetitive tasks in a challenging work environment. The impact becomes even more dramatic when you consider that one such company faced with this challenge was experiencing staff turnover in their production area of nearly 25%!
Kits (single-use disposables)
Advanced therapy companies often don’t associate manual processes with kits but within a manual process there are several small single-use kits and components that represent nearly 20% of the COGs. Many of these consumables will also be proprietary and difficult to negotiate substantial price reductions on and hence don’t change much at scale.
Production clean rooms
When you add together the cost of running and maintaining a class B processing facility with the amortized cost of building and qualification, the cost of production clean rooms is approximately 10% of the COGs. Whilst the relationship between the floor area of class B processing suites and the indirect facility required to support the production isn’t linear, it also isn’t trivial. However, for simplicity we have not included indirect facility costs in the model. The model is intended to provide guidance on appropriate strategies and technologies for scale-up/out. For this reason we have also assumed that the production clean rooms are a greenfield installation.
QC (including labor, raw materials & capital amortization)
Because QC is only 10% of the COGs and because the challenges of managing large numbers of QC samples every day don’t become obvious until patient numbers increase, QC is often not given adequate consideration. The range of QC tests being performed and the equipment and number of personnel required to perform the tests will need to be addressed.
If we now reflect on which of these items can be changed without a significant comparability risk, it becomes clear that most of the COGs are locked in before clinical trials even start.
So, where to from here for COGs?
In razor–razor blade systems, it’s the razor blade that hurts
Reagents represent perhaps the greatest challenge and opportunity to significantly reducing the cost of CAR-T therapies:
- They are the single largest contributor to COGs
- They are selected very early in the development cycle, often pre-IND
- They are fundamental to defining the product and hence difficult to change
- There are few options available
- The easy options are not necessarily commercially viable long term
I once stated that the best consumables are no consumables (unless you are a supplier) since their cost passes directly through to every single patient you treat. Significant businesses across many industries have been created off the back of these razor–razor blade models. Although they clearly satisfy a market need, it relies on the razor blade being reasonably priced and produced in sufficiently high volumes to leverage economies of scale.
Within the CAR-T market, we are already seeing significant differences in the processes and reagents being used to develop therapies, due in part at least to the need to reduce the COGs.
Longer term, as the advanced therapy market continues to evolve and mature, I expect to see considerable effort directed towards alternative technologies that substantially reduce the cost and usage of reagents and kits. In the mean time, many companies will be depending on modest shifts in consumable costs through economies of scale and competition.
Why you must close your process
Scale-out using a class B process suite for every patient is not practical
The issues associated with scale-out based on manual, open process using class B process suites has been understood for some time:
- Class B clean room space is expensive to build, operate and maintain;
- Facility utilization is low with significant batch changeover time to minimize the risk of contamination;
- Movement of personnel between class B suites processing different batches is time consuming making it very difficult to share resources and optimize staff utilization;
- Major investment and management challenge as scale-out progresses with additional capacity requiring additional construction and qualification of clean rooms.
It is commonly accepted that this approach to scale-out is simply impractical (Figure 4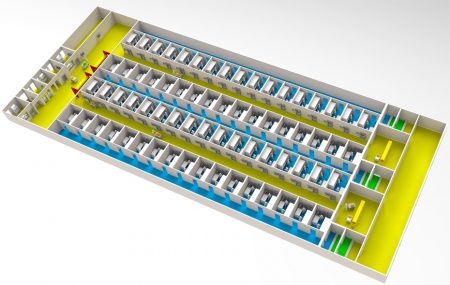
Functional closure of the entire process within the production area is critical to realizing clean room cost savings
What often isn’t fully appreciated is that in order to realize the facility and operational cost benefits, you need to process multiple batches in parallel within a single clean room, preferably class C. Assuming that this is achieved,we greatly simplify the facility and reduce the clean room capital amortization and operating costs from 10% of the COGs to only 1% (Figure 5
However, this is only possible if you can demonstrate batch segregation, which requires functional closure of every single process step taking place in your production area. This includes any time you add to or remove material from the closed system, for example media exchange during incubation and QC sample isolation.
Closure of individual processes alone is not enough and achieving functional closure of systems that were not designed with flexibility in mind is a non-trivial task. It requires a comprehensive approach to the design of your process including automated process technologies and single-use kits that can be integrated and optimized to suit your process.
Automation is critical to commercial production & an enabler of closed processing
It isn’t practical to talk about the benefits of closed processing without also discussing automation. Automation is clearly an enabler of closed processing but it is also critical for commercial scale, cGMP manufacture. Automation requires a system level approach to ensure that the technologies and the level of automation applied are appropriate for your business. Importantly, the ideal solution for clinical trials isn’t necessarily the best solution for commercial scale manufacture. At the simplest level, research and early-stage clinical trials requires flexible solutions since the process is still in flux. A higher COG at such low patient numbers is also of lesser importance than proving that the therapy works. Conversely, commercial manufacture requires that the process is cost effective and reproducible.
Choosing a suitable scale-up strategy for your advanced therapy is a complex process. It is influenced by the decisions you have already made and the commercial and regulatory constraints within which your business must operate. Carefully planning what, how and when you will implement automation is critical.
Process integrity is king
“cGMPs provide for systems that assure proper design, monitoring and control of manufacturing processes and facilities. Adherence to the cGMP regulations assures the identity, strength, quality and purity of drug products by requiring that manufacturers of medications adequately control manufacturing operations. This includes establishing strong quality management systems, obtaining appropriate quality raw materials, establishing robust operating procedures, detecting and investigating product quality deviations, and maintaining reliable testing laboratories. This formal system of controls at a pharmaceutical company, if adequately put into practice, helps to prevent instances of contamination, mix-ups, deviations, failures and errors. This assures that drug products meet their quality standards.” [2].
A robust cGMP process includes the following:
- Identification of critical control points in the manufacturing process that affect product characteristics
- In-process controls including critical control points and in-process testing
We define ‘process integrity’ as the ability of a system to perform the same process for every batch, at every site, for every patient, every time. In doing so, we help to ensure that patient safety and product quality are not compromised. A key strategy to achieving process integrity is to eliminate process hazards such as:
- Close the process to minimize the risk of contamination and provide clear batch segregation
- Automate manual tasks to eliminate hazards and reduce the likelihood of occurrence, for example:
- Batch record creation
- Fluid transfer, formulation and homogeneity
- Sample isolation
- Barcodes or RFID for chain of custody and identity
Removal of operator dependent process times
Automation incorporating these features is key to achieving and maintaining cGMP. However, as can be seen from Figure 6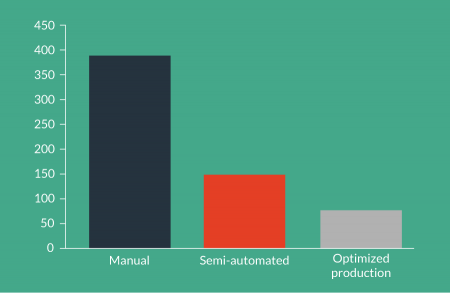
Don’t use your automated processing equipment as incubators
The optimal level of automation isn’t always obvious and the same logic for hazard reduction also applies to process integration. Functional closure needs to be assured but this does not imply that every single process must be integrated into a single piece of automation and a single kit.
Take incubation, for example. Within a typical CAR-T process we would expect several days of incubation during which time the product may only require monitoring and periodic addition of media. Consequently, traditional incubation in single use vessels can be both an appropriate and cost effective strategy.
By contrast, the processes before and after incubation are quite complex and may be time critical. Once automated, they can usually be completed relatively quickly (typically 1–4 hours per batch). Automation of these processes is also essential to ensure process integrity and significantly reduces the amount of labor required.
From our modeling, locking up the automation and using it as an incubator would result in:
- 10 times the number of processing machines
- 4 times the Clean Room production area
- 4 times the capital investment
- 20% increase in COGs
Using your automated processing system as an incubator is not necessary, cost effective or appropriate for commercial scale production (Figure 7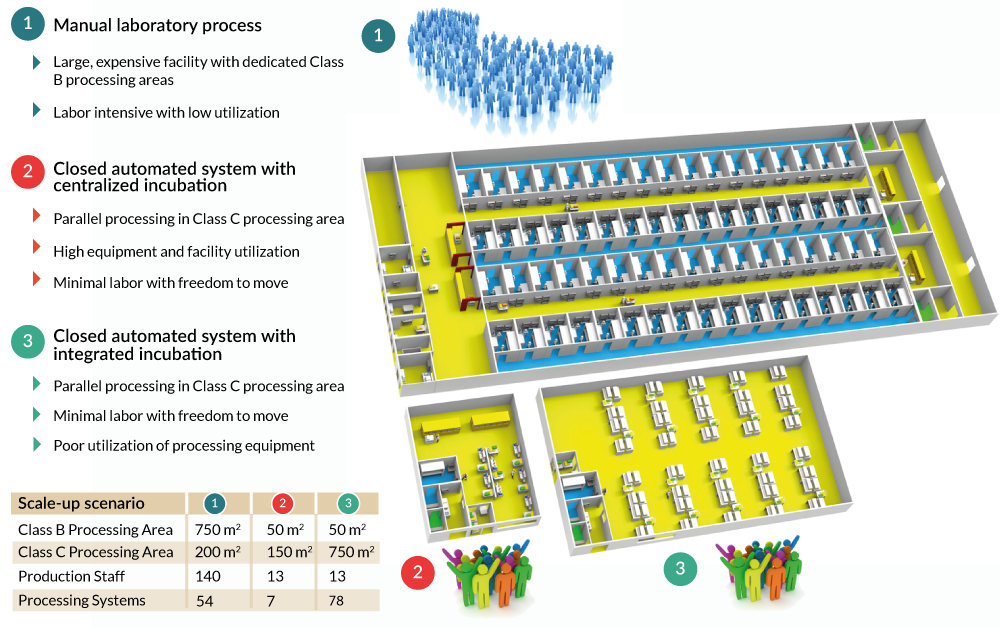
Despite what your CFO thinks, labor isn’t just a COGs issue
There is no avoiding the fact that a manual CAR-T process is very labor intensive and hence prone to error. Whilst it could be made to comply with cGMP, it would be a significant management challenge and would not be cost effective.
A manual CAR-T process would depend on highly trained and qualified operators to consistently perform repetitive tasks in an inconvenient class B environment. Hiring, training and retaining a capable production team would be a very significant challenge not to mention the indirect labor and facility costs required to support them.To be blunt, it isn’t a practical scale-out strategy for CAR-T therapies.
As has been proven across many industries, automation can have a profound impact on both the COGs and quality/process integrity. Interestingly, despite automation projects often being justified based on labor saving, because it’s usually easy to calculate, it is generally recognized that the greatest benefit is actually the improvement in quality/process integrity.
That said, for a CAR-T process, we estimate that automation can deliver labor savings of >75% and will provide a very attractive return on investment.
Putting it all together
The advanced therapy industry has philosophically moved a long way in the past decade, from a common belief that autologous therapies would never be commercially viable to now knowing that it is possible.
In Figure 8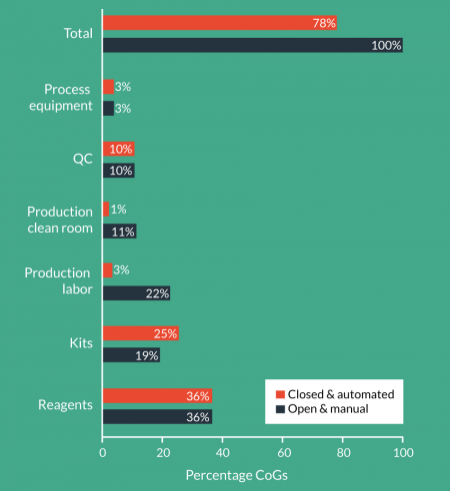
Even more significant is that we have not attempted to address the cost of reagents, since in the large part we are engaged after the reagents are substantially defined.
As stated previously, I expect that we will see significant R&D taking place surrounding the cost and consumption of reagents as the advanced therapy market continues to mature and grow.
The value of investing in such solutions is profound at commercial scale and if commenced early, usually provides a payback within the clinical trials period.
A lot of progress is being made but there are significant opportunities to further reduce the COGs.
Comparability is a much bigger problem than you think
A typical CAR-T system must perform several processes that are critical to quality and that define your product including T-cell selection and activation, genetic modification, expansion, washing, concentration, formulation and fill and finish.
Whilst there are many commonalities, some of the most fundamental requirements demanded of a commercial manufacturing process are in direct conflict with those needed during early stage clinical trials:
- Early-stage clinical trials require reagents and equipment that are flexible, adaptable, affordable and readily available
- Commercial production requires solutions that are reproducible, optimized, scalable and transferable
Managing the transition between these two paradigms without putting existing clinical data at risk, is one of the most critical factors to commercial success. Proving comparability between the preferred commercial process and that used in clinical trials will be inevitable.
According to ICH Q5E, “a comparability exercise should provide analytical evidence that a product has highly similar quality attributes before and after manufacturing process changes, with no adverse impact on safety or efficacy, including immunogenicity”.
The challenges faced by advanced therapy companies in early clinical trials are typically:
- Milestones must be met to gain the next round of funding;
- Funding is tight;
- The process is in flux;
- The resources required for process development are not available (usually wrestling with making product for clinical trials using the very same manual process that needs to be replaced).
Many companies, therefore, opt to not address the comparability risk during early trials with the belief that it can be sorted out during subsequent clinical trials or even post-approval with a comparability assessment or bridging study. Most would not contemplate repeating clinical trials due to the significant cost and time impact. What often isn’t appreciated is that many of the process changes contemplated or required for commercial production, are far from trivial and may not be practical or possible.
It is important to learn from those that have paved the way on comparability and not just those in the advanced therapy field. I recall a poster on a wall 20 years ago that simply stated, “Your poor planning is not my emergency”. As specialists in automation and manufacturing we can always help; however, the later you leave it the greater the constraints within which we will have to work.
Choosing a scale-up strategy
Manual, open processes in BSCs are convenient for clinical trials but they are not suitable for commercial scale production.
Investing in the development of a fully automated, optimized system for your process prior to regulatory approval is a significant, at-risk investment and unlikely to be a practical option for most companies.
Choosing a suitable scale-up strategy for your cell therapy is, therefore, a complex process. It is influenced by the decisions you have already made and the commercial and regulatory constraints within which your business must operate. Scinogy’s approach to scale-up is based on a comprehensive understanding of your process and your business needs. The solutions we provide are then guided by the following principles:
- Minimize schedule impact by applying technologies and comparable processes previously used unless impractical for commercial production.
- Ensure it is an affordable and flexible system suitable for clinical trials that can be also be used for commercial cGMP production.
- Focus on eliminating process hazards critical to patient safety and product quality.
- Incorporate process monitoring and control of critical process parameters with automated data capture.
- Minimize the requirement for BSCs and class A/B clean rooms by closing the process.
- Achieve a reasonable COG at launch with the ability to optimize as capacity increases.
Figure 9 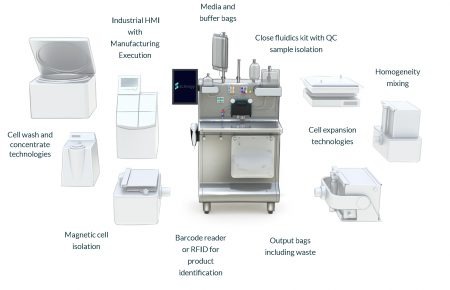
In addition to safety and efficacy, turning your clinical trials into commercial reality requires that your therapy is also:
- Reproducible,
- Scalable
- Transferable
- Commercially viable
Remember this as you plan your clinical trials and make sure that you have allocated sufficient time, resources and funds to address the challenge.
TRANSLATIONAL INSIGHT
There is no doubting that automation delivers significant cost benefits but this is only part of the story and in many ways not the most important benefit. Automated, closed manufacturing systems specifically configured for your process and business needs are also:
- Reproducible, providing confidence that your process is performed the same way every batch, every time and that clinical data generated is dependable
- Critical to achieving and maintaining cGMP during clinical trials and commercial production
- Commercially justified at relatively few patients per year and usually before clinical trials are complete
- Enablers of rapid and predictable capacity expansion once approval is granted
- A clear demonstration to investors and regulators of process and manufacturing maturity
It is always easier to find excuses for why something cannot be done:
- Insufficient funds
- Not enough time
- Not enough resources
- Process not sufficiently defined
Ironically if this approach had been taken 10 years ago, autologous therapies that are currently driving the advanced therapy market such as CAR-T, may not exist today.It is important that anyone seriously contemplating the advanced therapy industry be planning for success. That means applying appropriate cGMP manufacturing equipment and processes during clinical trials that set you up for commercial production. Don’t allow short-term gain to become your long-term pain.
AFFILIATION
David James
CEO, Scinogy Pty Ltd, Australia
E-mail: david.james@scinogy.com
FINANCIAL & COMPETING INTERESTS DISCLOSURE
The author is CEO of Scinogy Pty Ltd. No writing assistance was utilized in the production of this manuscript
REFERENCES
1. FDA, Guidance for FDA Reviewers and Sponsors – Content and Review of Chemistry, Manufacturing, and Control (CMC) Information for Human Gene Therapy Investigational New Drug Applications (IND’s), 2008
2.FDA, Facts About the Current Good Manufacturing Practices (CGMPs), 2015