Disruptive bench-scale purification of lentivirus using affinity-liquid phase separation technology
Cell & Gene Therapy Insights 2023; 9(9), 1255–1269
DOI: 10.18609/cgti.2023.163
Lentiviral vectors are widely used in cell and gene therapy, but their high production costs hamper early-stage research efforts. Furthermore, many concentration and purification methods available to early-stage researchers today are difficult to scale or lack specificity for lentiviral purification, hindering preclinical research and translation to clinical and commercial scales. We have developed a research-scale lentiviral reagent with the potential to transform lentiviral purification workflows. The reagent is quick and easy to use, yields high titer lentiviral vectors, and, because of its specificity, is more effective at contaminant removal than other LV purification products designed for research use. By specifically attaching to the viral envelope in solution and then forming liquid droplets around the lentivirus, IsoTag™ LV protects viruses from aggregation and degradation while dramatically increasing the effective size and density of the LV, thus facilitating easy separation from other smaller and less dense contaminants (i.e., host cell proteins). The actual process of capture is very simple and can be implemented by any user, at any skill level, with low-cost lab equipment. The design and development of a small-scale centrifugation process and novel reagent detailed herein lays the foundation for additional development of the IsoTag™ LV for larger scale processing. IsoTag™ LV is poised to democratize LV production and purification in the research world and accelerate development of new cell and gene therapies.
Introduction
Lentiviral vectors (LVs) are widely used viral vectors necessary for the production of several commercial cell and gene therapies [1]Bulcha JT, Wang Y, Ma H, et al. Viral vector platforms within the gene therapy landscape. Sig. Transduct. Target Ther. 2021; 6, 53.. However, their innate fragility and cytotoxicity, due to their lipid bilayer and VSVG (vesicular stomatitis virus envelope glycoprotein) pseudotype, respectively, means that the manufacturing cost of these vectors is extremely high [2]Kumru OS, Wang Y, Gombotz CWR, et al. Physical characterization and stabilization of a lentiviral vector against adsorption and freeze-thaw. J. Pharm. Sci. 2018; 107, 2764–2774. [3]Moreira AS, Cavaco DG, Faria TQ, Alves PM, Carrondo MJT, Peixoto C. Advances in Lentivirus Purification. Biotechnol. J. 2021; 16, 2000019. [4]Rahman H, Taylor J, Clack B, Stewart RS, Canterberry SC. Effects of storage conditions on the morphology and titer of lentiviral vectors. Tex. J. Microsc. 2013; 44, 976–988. [5]Carmo M, Alves A, Rodrigues AF, et al.Stabilization of gammaretroviral and lentiviral vectors: from production to gene transfer. J. Gene Med. 2009; 11, 670–678.. Traditional downstream purification strategies are being commercially adapted for large and complex viruses [3]Moreira AS, Cavaco DG, Faria TQ, Alves PM, Carrondo MJT, Peixoto C. Advances in Lentivirus Purification. Biotechnol. J. 2021; 16, 2000019. [6]Merten OW, Hebben M, Bovolenta C. Production of lentiviral vectors. Mol. Ther. Methods Clin. Dev. 2016; 3, 16017. [7]Valkama AJ, Oruetxebarria I, Lipponen EM, et al. Development of Large-Scale Downstream Processing for Lentiviral Vectors. Mol. Ther. Methods Clin. Dev. 2020; 17, 717–730.. However, these methods lack standardization, require significant optimization, and have suboptimal product recovery, thereby contributing to the high development costs for lentivirus-based gene therapies. As such, potentially curative treatments and therapies are often prohibitively expensive, in some cases costing over one million USD per dose [8]Comisel R, Kara B, Fiesser FH; Farid SS. Lentiviral vector bioprocess economics for cell and gene therapy commercialization. Biochem. Eng. J. 2021; 167, 107868.. Early-stage research on these therapies typically begins in academic laboratories working at small scale [9]Saenz DT, Loewen N, Peretz M, et al. Unintegrated lentivirus DNA persistence and accessibility to expression in nondividing cells: analysis with class I integrase mutants. J. Virol. 2004; 78(6), 2906–2920. [10]Nguyen H, Kirkton R, Bursac N. Engineering prokaryotic channels for control of mammalian tissue excitability. Nat. Commun. 2016; 7, 13132. here defined as 1 L or less and termed ‘research-scale’. Research-scale production typically relies on ultracentrifugation [11]Brown LY, Dong W . Kantor B. An improved protocol for the production of lentiviral vectors. STAR Protoc. 2020; 1(3), 100152. or low-speed centrifugation using PEGprecipitation. Little has been done to improve upon the concentration, purity, or scalability of these methods, despite their criticality to the early-stage development of advanced therapies. Standardized downstream purification of LV at the research-scale, especially with methods improving high-throughput and quality, would improve the reliability of the vector, increase the speed of preclinical research, and ease the translation of new technologies to clinical and commercial scales.
Research-scale LV is most commonly produced using a triple transfection protocol [11]Brown LY, Dong W . Kantor B. An improved protocol for the production of lentiviral vectors. STAR Protoc. 2020; 1(3), 100152. in an adherent or suspension cell line with a customized cocktail of additives. Following harvest, researchers concentrate and purify their LV through an assortment of methods that vary in cost, efficacy, scalability, and ease of operation. Unfortunately, no single method or product provides both rapid concentration and contaminant removal, but common methods are outlined herein. Standard concentration and purification methods available to researchers involve ultracentrifugation, low speed centrifugation, and filtration [6]Merten OW, Hebben M, Bovolenta C. Production of lentiviral vectors. Mol. Ther. Methods Clin. Dev. 2016; 3, 16017. [12]Soldi M, Sergi LS, Unali G, et al. Laboratory-scale lentiviral vector production and purification for enhanced ex vivo and in vivo genetic engineering. Mol. Ther. Methods Clin. Dev. 2020; 19, 411–425.. Briefly, ultracentrifugation involves layering crude LV harvest material over a sucrose gradient and using ultracentrifugation to separate LV particles from the viral supernatant. This method is technically challenging, requires costly equipment, and it is not scalable. Many researchers choose simply to centrifuge their harvest material at low speed to pellet and remove large cellular debris, but this method does not concentrate the LV, nor does it remove smaller contaminants and dsDNA that can interfere with downstream applications [6]Merten OW, Hebben M, Bovolenta C. Production of lentiviral vectors. Mol. Ther. Methods Clin. Dev. 2016; 3, 16017. [13]Kutner RH, Puthli S, Marino MP, Reiser J. Simplified production and concentration of HIV-1-based lentiviral vectors using HYPERFlask vessels and anion exchange membrane chromatography. BMC Biotechnol. 2009; 9, 10.. As the importance of creating a high-quality LV product that can be reproducibly used for experiments has recently been emphasized [6]Merten OW, Hebben M, Bovolenta C. Production of lentiviral vectors. Mol. Ther. Methods Clin. Dev. 2016; 3, 16017. [12]Soldi M, Sergi LS, Unali G, et al. Laboratory-scale lentiviral vector production and purification for enhanced ex vivo and in vivo genetic engineering. Mol. Ther. Methods Clin. Dev. 2020; 19, 411–425., it is important to consider contaminant removal even at small scales.
To create quality LV, one group proposed mimicking commercial scale approaches to LV manufacturing [12]Soldi M, Sergi LS, Unali G, et al. Laboratory-scale lentiviral vector production and purification for enhanced ex vivo and in vivo genetic engineering. Mol. Ther. Methods Clin. Dev. 2020; 19, 411–425.Soldi M, Sergi LS, Unali G, et al. Laboratory-scale lentiviral vector production and purification for enhanced ex vivo and in vivo genetic engineering. Mol. Ther. Methods Clin. Dev. 2020; 19, 411–425. involving a complex set of steps requiring equipment similar to that used in clinical-scale manufacturing suites. With this method, they were able to reduce DNA contaminants by 1log with total LV recoveries of around 20–40% [12]Soldi M, Sergi LS, Unali G, et al. Laboratory-scale lentiviral vector production and purification for enhanced ex vivo and in vivo genetic engineering. Mol. Ther. Methods Clin. Dev. 2020; 19, 411–425.Soldi M, Sergi LS, Unali G, et al. Laboratory-scale lentiviral vector production and purification for enhanced ex vivo and in vivo genetic engineering. Mol. Ther. Methods Clin. Dev. 2020; 19, 411–425.. Unfortunately, this protocol requires expensive equipment and highly skilled operators better suited for a vector core than an individual researcher or laboratory, and thus is inaccessible to many laboratory-scale LV producers. Other commercially available options provided in kit formats that employ common laboratory equipment are more accessible to individual researchers or research groups. Many of these kit-type offerings rely on PEG precipitation to concentrate LV from harvest material, leaving the PEG reagent in the final LV product and creating large pellets that limit final concentration. While these reagents are non-toxic and may protect the virus during freeze-thaw [14]PEG-it Virus Precipitation Solution. System Biosciences. it has been demonstrated that PEG monomer contaminants and breakdown products can potentially be cytotoxic or limit in vivo use due to their non-biodegradability and antigenicity [15]Liu G, Li Y, Yang L, et al. Cytotoxicity study of polyethylene glycol derivatives. RSC Adv. 2017; 7, 18252–18259. [16]Thi TTH, Pilkington EH, Nguyen DH, Lee JS, Park KD, Truong NP. The Importance of Poly(ethylene glycol) Alternatives for Overcoming PEG Immunogenicity in Drug Delivery and Bioconjugation. Polymers 2020; 12(2), 298. [17]Gaberc-Porekar Zore I, Podobnik B, Menart V. Obstacles and pitfalls in the PEGylation of therapeutic proteins. Curr. Opin. Drug Discov. Devel. 2008; 11(2), 242–250.. Other options include magnetic bead sorting, but use of this reagent requires the purchase of accessories such as a magnetic tube rack specific to each tube size and cannot be easily scaled up. Kits offer specific benefits to research scale LV production including simplicity, speed, protection during storage, and removal of the concentrating reagent. However, no single method provides a combination of all these benefits in an ultimate, easy-to-use product for research scale users. We sought to design a product that offered maximum flexibility to the user by demonstrating compatibility across broad feed streams and wide titer ranges with a protocol that is rapid and reproducibly yields high-quality LV.
To that end, we designed and demonstrated proof-of-concept on a research-scale LV purification reagent. The LVspecific reagent, termed IsoTag™ LV, is a recombinant protein comprising an LVspecific affinity ligand combined with Isolere’s proprietary bioinspired biopolymer that provides tuneable and reversible liquid–liquid phase separation behavior [18]Haley J, Jones JB, Petraki S, et al. IsoTag™ 285 AAV: an innovative, scalable and non-chromatographic method for streamlined AAV manufacturing. Cell Gene Ther. Insights 2022; 8, 1287–1300.. The tuneable phase separation behavior arises from the stimulus-responsive biopolymer, whose phase separation is triggered by an external environmental cue, such as shift in temperature, pH, or conductivity [19]Meyer D, Chilkoti A. Purification of recombinant proteins by fusion with thermally-responsive polypeptides. Nat. Biotechnol. 1999; 17, 1112–1115.. The IsoTag™ LV process described herein was designed to undergo reversible phase transition with the modulation of sodium chloride at ambient temperature. However, temperature manipulation could be used in place of conductivity shift. The process is illustrated in the Figure 1 schematic: IsoTag™ binds in solution to a vector, pulls the vector into a droplet upon the addition of the environmental trigger, and then releases the vector under elution conditions. Vector purification occurs in the droplet phase—the droplets are much denser than the remaining particles and thus can be separated by low-speed centrifugation. The droplets could also be separated on the basis of size with microfiltration. While a scalable microfiltration method is outside the scope of this publication, it is under development and will be the subject of future publications. Of note, the cartoons in Figure 1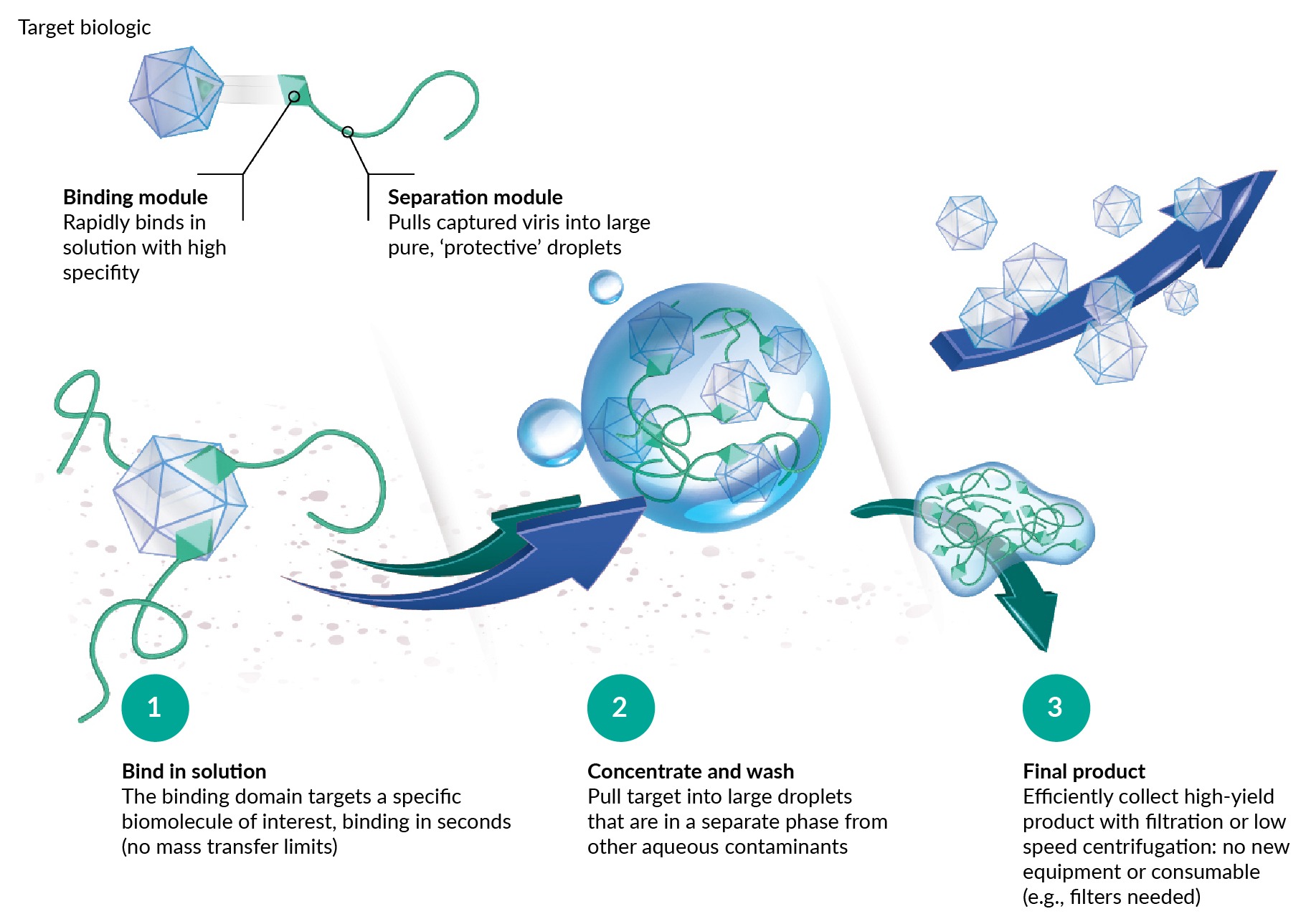 IsoTag™ process schematic.Visual representation of droplet formation and vector sequestering with the IsoTag™ LV reagent. are not drawn to scale, IsoTag™ proteins are roughly 50 kD in size in their soluble state, so much smaller than the vector they are shown to capture. Furthermore, the droplets are 1–10 µm in size, much larger relative to the vector than shown in Figure 1. Lastly, Figure 1 serves to illustrate the general concept of purification using IsoTag™ reagents, herein we describe a process to capture and concentrate LV without removing the IsoTag™ LV reagent, emphasizing simplicity and speed for small-scale researchers.
IsoTag™ process schematic.Visual representation of droplet formation and vector sequestering with the IsoTag™ LV reagent. are not drawn to scale, IsoTag™ proteins are roughly 50 kD in size in their soluble state, so much smaller than the vector they are shown to capture. Furthermore, the droplets are 1–10 µm in size, much larger relative to the vector than shown in Figure 1. Lastly, Figure 1 serves to illustrate the general concept of purification using IsoTag™ reagents, herein we describe a process to capture and concentrate LV without removing the IsoTag™ LV reagent, emphasizing simplicity and speed for small-scale researchers.
The IsoTag™ LV reagent process delivers highly selective capture of LV particles by employing a de novo-designed affinity binding domain that interacts with the VSVGs. Because of this, the IsoTag™ LV reagent could likely bind to and purify any VSVG pseudotyped particle, however, the focus of our development was on VSVG pseudotyped lentiviral purification. Combining the affinity capture and phase-separating phenomena, this purification method is herein referred to as affinity liquid phase separation via centrifugation (ALPS-CF). Unlike the non-specific capture methods available today, the high specificity of our reagent enables exquisitely selective capture of the target vectors. This results in higher yields and higher purity with a robust reagent agnostic to virus titer and feed material.
Results
The IsoTag™ LV reagent is simple to use: a small volume of reagent from a concentrated stock is mixed into harvest LV material (Figure 2A) before adding a phase transition buffer to pull LV particles into protective, phase-separated, virus-containing droplets (Figure 2B) that are isolated by centrifuging at low speed. The viral supernatant is then removed, and the virus-containing pellet is resuspended in the user’s buffer and volume of choice (Figure 2C). This process results in over 95% LV recovery, as visualized by the GFP+ infected cells (Figure 2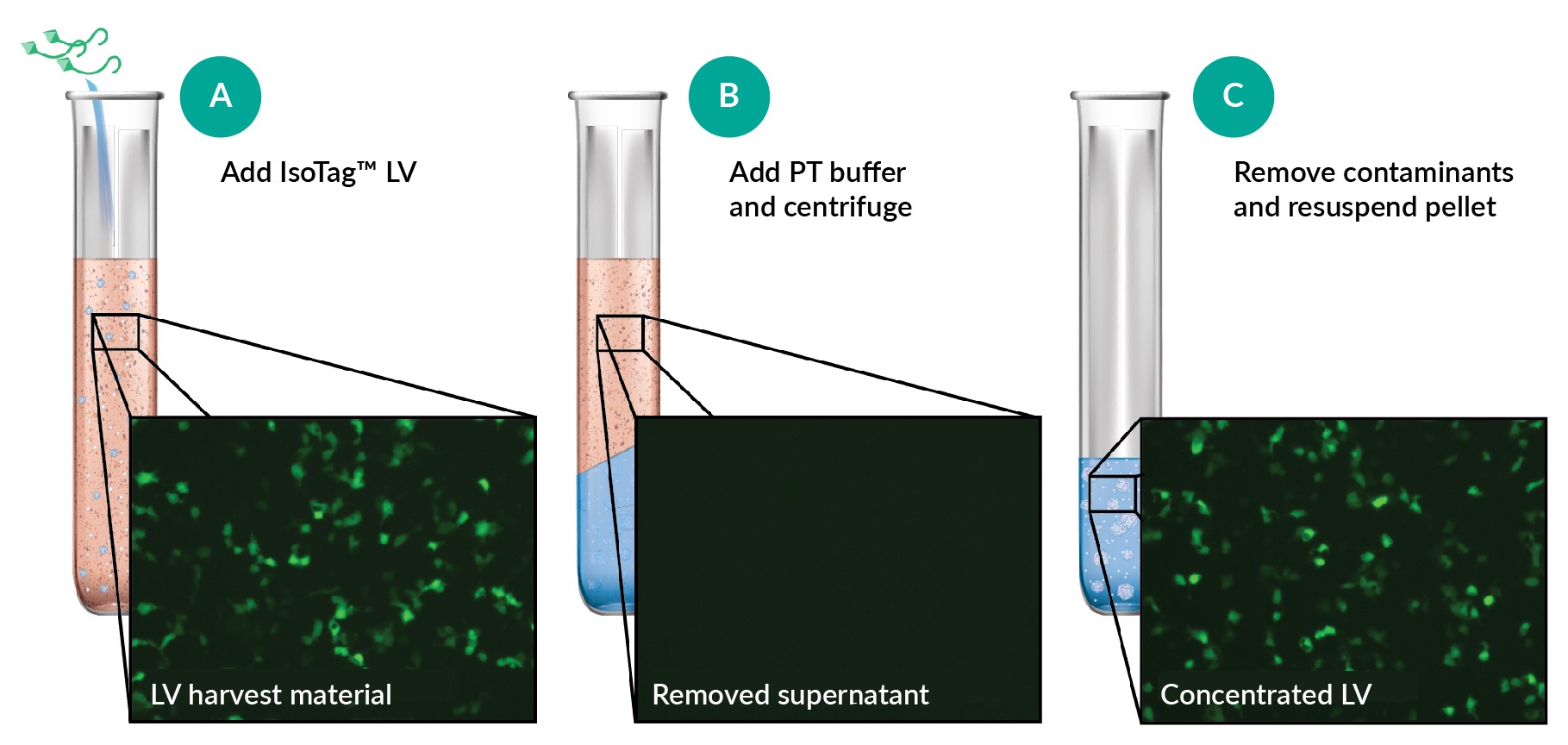 One step concentration and purification of crude lentiviral harvest material.Schematic illustrating LV purification by (A) the addition of IsoTag LV to crude harvest material, (B) droplet sequestering of LV with the addition of phase transition (PT) buffer, and (C) contaminant removal. Representative images of CMVLVeGFP transducing HEK293T cells at different stages of the purification process, diluted to identical volumes are shown in the pop-outs.). Because the IsoTag™ LV reagent is VSVG-specific, the concentrated virus has greatly reduced levels of contaminants even without a dedicated washing step.
One step concentration and purification of crude lentiviral harvest material.Schematic illustrating LV purification by (A) the addition of IsoTag LV to crude harvest material, (B) droplet sequestering of LV with the addition of phase transition (PT) buffer, and (C) contaminant removal. Representative images of CMVLVeGFP transducing HEK293T cells at different stages of the purification process, diluted to identical volumes are shown in the pop-outs.). Because the IsoTag™ LV reagent is VSVG-specific, the concentrated virus has greatly reduced levels of contaminants even without a dedicated washing step.
Initial work involved evaluation of the IsoTag™ LV reagent’s ability to bind and concentrate LV, while simultaneously removing host cell proteins and other contaminants. As the ALPS-CF process is performed on a volumetric basis, the protocol is fixed regardless of the starting titer. In these first experiments, the 50 mL of removed supernatant waste was replaced with 1 mL of PBS easily. The pellet, resuspended in this 1 mL of PBS, yields purified LV that is concentrated 50-fold. When the IsoTag™ LV reagent was used to concentrate crude LV from adherent HEK293T cells (with no additional clarification or nuclease treatment steps), we were able to concentrate 50-fold to a functional titer of 5.3e10 GFU/mL (Figure 3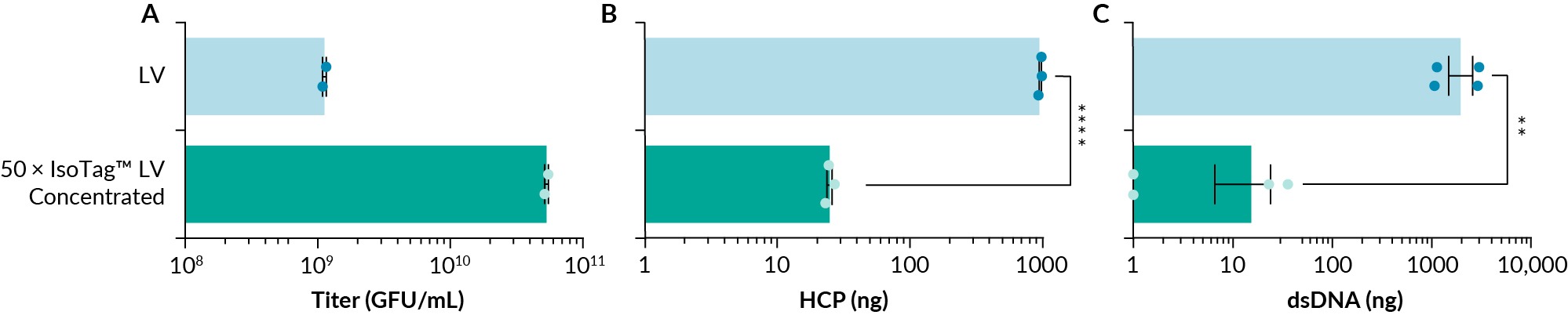 IsoTag™ LV is a successful LV concentration reagent with excellent contaminant removal.(A) IsoTag™ LV was able to concentrate LV 50-fold to 5.3e10 GFU/mL and concentration resulted in a (B) 1.5-log reduction in host cell protein (HCP) to 24.9 ng and a (C) 2-log reduction in dsDNA contaminants to 15.2 ng. Undetectable dsDNA samples were given a value of 1. n=2–6, error bars represent the standard error of the mean. Statistical significance calculated using a (A & B) one-way ANOVA or (C) student t-test. ns=not significantly different. **p<0.01; ****p<0.0001.A). Functional recoveries of over 98% were observed (Figure S1D). This indicated the IsoTag™ LV reagent bound and captured nearly all the crude LV in solution. Without the need to add a dedicated wash step in the protocol, concentrating crude LV using the IsoTag™ LV reagent also resulted in a 1.5-log reduction of the contaminating host cell proteins to 25 ng (Figure 3B), and a 2-log reduction in dsDNA contaminants to 15.2 ng, with two of the replicates below the detection limit (Figure 3C). This is because the IsoTag™ LV reagent contains an affinity ligand to bind specifically to LV particles, thus avoiding precipitation of other unwanted contaminants and impurities. Similar to other reagents on the market today, the IsoTag™ LV reagent remains in the final product with the concentrated LV.
IsoTag™ LV is a successful LV concentration reagent with excellent contaminant removal.(A) IsoTag™ LV was able to concentrate LV 50-fold to 5.3e10 GFU/mL and concentration resulted in a (B) 1.5-log reduction in host cell protein (HCP) to 24.9 ng and a (C) 2-log reduction in dsDNA contaminants to 15.2 ng. Undetectable dsDNA samples were given a value of 1. n=2–6, error bars represent the standard error of the mean. Statistical significance calculated using a (A & B) one-way ANOVA or (C) student t-test. ns=not significantly different. **p<0.01; ****p<0.0001.A). Functional recoveries of over 98% were observed (Figure S1D). This indicated the IsoTag™ LV reagent bound and captured nearly all the crude LV in solution. Without the need to add a dedicated wash step in the protocol, concentrating crude LV using the IsoTag™ LV reagent also resulted in a 1.5-log reduction of the contaminating host cell proteins to 25 ng (Figure 3B), and a 2-log reduction in dsDNA contaminants to 15.2 ng, with two of the replicates below the detection limit (Figure 3C). This is because the IsoTag™ LV reagent contains an affinity ligand to bind specifically to LV particles, thus avoiding precipitation of other unwanted contaminants and impurities. Similar to other reagents on the market today, the IsoTag™ LV reagent remains in the final product with the concentrated LV.
As some of these competing products claim to provide additional storage benefits during freeze–thaw [14]PEG-it Virus Precipitation Solution. System Biosciences. we investigated the extent to which the IsoTag™ LV reagent could provide thermostabilizing properties to the LV particles. This effect is of particular interest to researchers because it creates a more reliable final product and easier storage and development workflows. Over the various conditions we tested, crude LV recoveries improved by 50–90% when the IsoTag™ LV reagent was present as an additive (Figure 4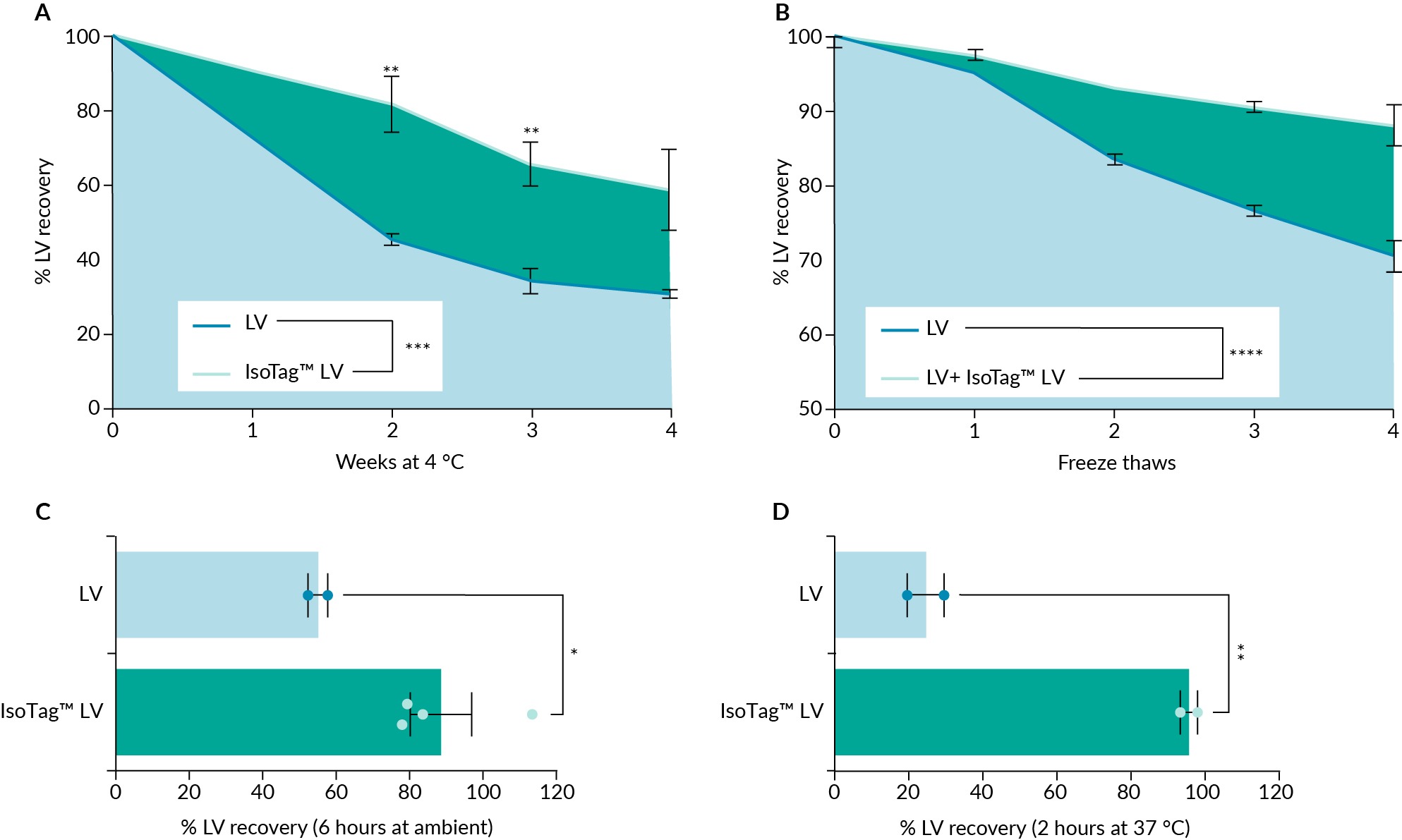 IsoTag™ LV addition significantly improves LV stability.The presence of IsoTag™ LV reagent resulted in a significant improvement in functional LV when stored at (A) 4 °C for 4 weeks (60% functional recovery), (B) after 4 freeze-thaw cycles (88% functional recovery), (C) after 6 h at ambient temperature (20–25 °C) (88.6% functional recovery), and (D) after 2 h at 37 °C (95.7% functional recovery). n=2–3, error bars represent the standard error of the mean. Statistical significance calculated using a (A & B) two-way ANOVA with Fishers LSD post hoc test or (C & D) students t-test. *p<0.05; **p<0.01.). From a starting titer of 1.6e7 GFU/mL, a functional recovery of 60% was obtained when storing crude LV at 4–8 °C with IsoTag™ LV reagent for one month, compared to 30% recovery for LV alone in the same storage conditions without IsoTag™ LV reagent (Figure 4A, Figure S2A). This data demonstrates a two-fold improvement in LV stability, and the trend is consistent across weekly timepoints. Furthermore, crude LV samples stored at –80 °C containing IsoTag™ LV additive had significant improvement in recoveries of 88% functional recovery after three or more freeze-thaw cycles when compared to LV alone (Figure 4B & Figure S2D). When left at ambient temperature (20–24 °C) for 6 h, a significant improvement in functional LV recovery of 88.6% was observed in IsoTag™ LV-containing samples compared to LV alone (Figure 4C & Figure S2B). Pushing this phenomenon even further, the IsoTag™ LV reagent improved functional LV recoveries over 4 × after a 2-h incubation at 37 °C with 95.7% functional recovery (Figure 4D, Figure S2C). Interestingly, when IsoTag™ LV reagent was mixed with the LV sample before the time 0 data point, an increase in the starting titer was noted (Figure S2). This was accounted for in all analyses by including a time 0 sample containing an LV and IsoTag™ LV reagent mixture to directly compare LV recoveries with an appropriate baseline. Taken together, these results highlight additional workflow advantages provided by the IsoTag™ LV reagent and its ability to stabilize these labile viruses in a variety of conditions.
IsoTag™ LV addition significantly improves LV stability.The presence of IsoTag™ LV reagent resulted in a significant improvement in functional LV when stored at (A) 4 °C for 4 weeks (60% functional recovery), (B) after 4 freeze-thaw cycles (88% functional recovery), (C) after 6 h at ambient temperature (20–25 °C) (88.6% functional recovery), and (D) after 2 h at 37 °C (95.7% functional recovery). n=2–3, error bars represent the standard error of the mean. Statistical significance calculated using a (A & B) two-way ANOVA with Fishers LSD post hoc test or (C & D) students t-test. *p<0.05; **p<0.01.). From a starting titer of 1.6e7 GFU/mL, a functional recovery of 60% was obtained when storing crude LV at 4–8 °C with IsoTag™ LV reagent for one month, compared to 30% recovery for LV alone in the same storage conditions without IsoTag™ LV reagent (Figure 4A, Figure S2A). This data demonstrates a two-fold improvement in LV stability, and the trend is consistent across weekly timepoints. Furthermore, crude LV samples stored at –80 °C containing IsoTag™ LV additive had significant improvement in recoveries of 88% functional recovery after three or more freeze-thaw cycles when compared to LV alone (Figure 4B & Figure S2D). When left at ambient temperature (20–24 °C) for 6 h, a significant improvement in functional LV recovery of 88.6% was observed in IsoTag™ LV-containing samples compared to LV alone (Figure 4C & Figure S2B). Pushing this phenomenon even further, the IsoTag™ LV reagent improved functional LV recoveries over 4 × after a 2-h incubation at 37 °C with 95.7% functional recovery (Figure 4D, Figure S2C). Interestingly, when IsoTag™ LV reagent was mixed with the LV sample before the time 0 data point, an increase in the starting titer was noted (Figure S2). This was accounted for in all analyses by including a time 0 sample containing an LV and IsoTag™ LV reagent mixture to directly compare LV recoveries with an appropriate baseline. Taken together, these results highlight additional workflow advantages provided by the IsoTag™ LV reagent and its ability to stabilize these labile viruses in a variety of conditions.
Next, to explore the broad utility of the ALPS-CF process, the IsoTag™ LV reagent binding performance was evaluated across a variety of conditions. Throughout these experimental conditions, over 98% of the functional crude LV was retained on average (Figure 5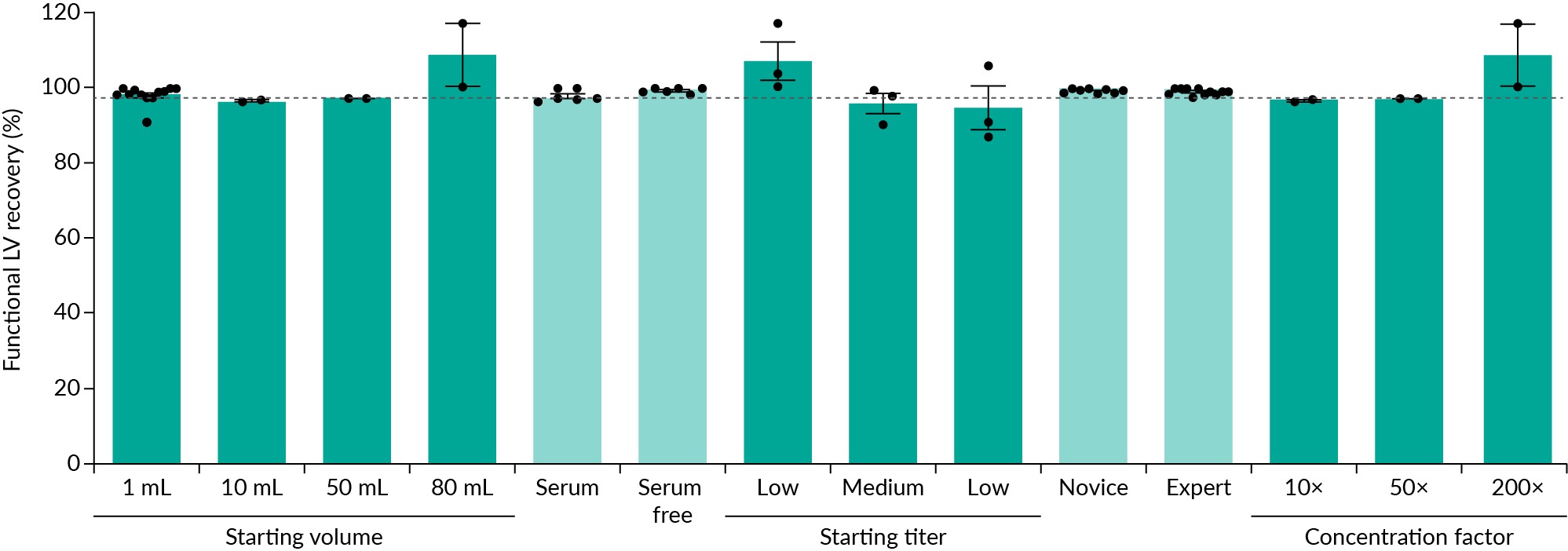 IsoTag™ LV is a robust LV concentration reagent.IsoTag™ LV reagent was used to concentrate LV under various conditions with an average functional recovery of 98% (gray line). High LV recovery was seen when using the IsoTag™ LV reagent regardless of starting volume, feed material, starting titer, experience level, or concentration factor. High, medium, and low, refer to functional starting titers of 1e8, 1e7, and 1e6 GFU/mL, respectively. n=2–12, error bars represent the standard error of the mean.). First, crude LV was concentrated from a variety of starting volumes (1–80 mL) and the total volume did not alter or interfere with the ALPS-CF process (Figure 5). 80 mL was the largest volume tested because it required the use of two 50 mL conical tubes, and it was assumed any larger volumes would be processed in a similar manner and thus have similar functional recoveries. IsoTag™ LV reagent binding performance was further examined with different feed streams, adherent production containing serum and serum-free suspension production, as they are known to have different contaminant profiles and binding efficiencies with other purification technologies. Again, the IsoTag™ LV reagent bound and concentrated over 98% of the LV regardless of feed stream (Figure 5). To ensure ALPS-CF is compatible across a wide range of LV harvest titers, binding with IsoTag™ LV reagent was examined with material titer from 1e6 to 1e8 GFU/mL. Titers following upstream production can be highly variable based on the size and complexity of the inserted gene [20]Yacoub N, al Romanowska M, Haritonova N, Foerster J. Optimized production and concentration of lentiviral vectors containing large inserts. J. Gene Med. 2007; 9, 579–584. and starting titer can have a dramatic impact on processing time and functional recoveries with other industry standard methods [3]Moreira AS, Cavaco DG, Faria TQ, Alves PM, Carrondo MJT, Peixoto C. Advances in Lentivirus Purification. Biotechnol. J. 2021; 16, 2000019. [6]Merten OW, Hebben M, Bovolenta C. Production of lentiviral vectors. Mol. Ther. Methods Clin. Dev. 2016; 3, 16017.. When the IsoTag™ LV reagent was mixed with high (1e8 GFU/mL), medium (1e7 GFU/mL), and low (1e6 GFU/mL) starting LV titers and used to capture LV, functional recovery titers were again above 97% (Figure 5). Furthermore, processing times were greatly reduced with the streamlined ALPS-CF process. While comparable kits take anywhere from 2–12 h to process LV material, using the IsoTag™ LV reagent took just 30 min.
IsoTag™ LV is a robust LV concentration reagent.IsoTag™ LV reagent was used to concentrate LV under various conditions with an average functional recovery of 98% (gray line). High LV recovery was seen when using the IsoTag™ LV reagent regardless of starting volume, feed material, starting titer, experience level, or concentration factor. High, medium, and low, refer to functional starting titers of 1e8, 1e7, and 1e6 GFU/mL, respectively. n=2–12, error bars represent the standard error of the mean.). First, crude LV was concentrated from a variety of starting volumes (1–80 mL) and the total volume did not alter or interfere with the ALPS-CF process (Figure 5). 80 mL was the largest volume tested because it required the use of two 50 mL conical tubes, and it was assumed any larger volumes would be processed in a similar manner and thus have similar functional recoveries. IsoTag™ LV reagent binding performance was further examined with different feed streams, adherent production containing serum and serum-free suspension production, as they are known to have different contaminant profiles and binding efficiencies with other purification technologies. Again, the IsoTag™ LV reagent bound and concentrated over 98% of the LV regardless of feed stream (Figure 5). To ensure ALPS-CF is compatible across a wide range of LV harvest titers, binding with IsoTag™ LV reagent was examined with material titer from 1e6 to 1e8 GFU/mL. Titers following upstream production can be highly variable based on the size and complexity of the inserted gene [20]Yacoub N, al Romanowska M, Haritonova N, Foerster J. Optimized production and concentration of lentiviral vectors containing large inserts. J. Gene Med. 2007; 9, 579–584. and starting titer can have a dramatic impact on processing time and functional recoveries with other industry standard methods [3]Moreira AS, Cavaco DG, Faria TQ, Alves PM, Carrondo MJT, Peixoto C. Advances in Lentivirus Purification. Biotechnol. J. 2021; 16, 2000019. [6]Merten OW, Hebben M, Bovolenta C. Production of lentiviral vectors. Mol. Ther. Methods Clin. Dev. 2016; 3, 16017.. When the IsoTag™ LV reagent was mixed with high (1e8 GFU/mL), medium (1e7 GFU/mL), and low (1e6 GFU/mL) starting LV titers and used to capture LV, functional recovery titers were again above 97% (Figure 5). Furthermore, processing times were greatly reduced with the streamlined ALPS-CF process. While comparable kits take anywhere from 2–12 h to process LV material, using the IsoTag™ LV reagent took just 30 min.
The ALPS-CF process is easy to implement in any laboratory outfitted with equipment basics. Six first-time users following the protocol without any additional prior training or guidance were able to recover greater than 98% of their crude LV using the ALPS-CF protocol. LV recoveries achieved by first-time users were indistinguishable from experienced users (Figure 5 & Figure S1E). New users will be able to readily adopt this ALPS-CF process for concentrating and purifying their crude LV vectors with consistent results for each lot on the first attempt. Last, the final concentration factor had little impact on the overall LV recoveries, again consistently meeting the 98% average when concentrating anywhere in the range of 10–200 × (Figure 5). Taken together, these data validate a robust and reproducible new method for LV recovery, concentration, and purification with IsoTag™ LV reagent.
Given the robust and simple implementation, we next sought to compare the IsoTag™ LV reagent with similar research grade, commercially available reagents. This testing was done both internally at Isolere’s laboratories and with an external partner, for further validation that the method can be successfully implemented without extensive experience with the underlying phase separation technology. When compared to a reagent that concentrates via PEG precipitation or magnetic bead separation, the IsoTag™ LV reagent produced more consistent recoveries across all starting titers and was the only reagent to consistently achieve 95% recovery (Figure 6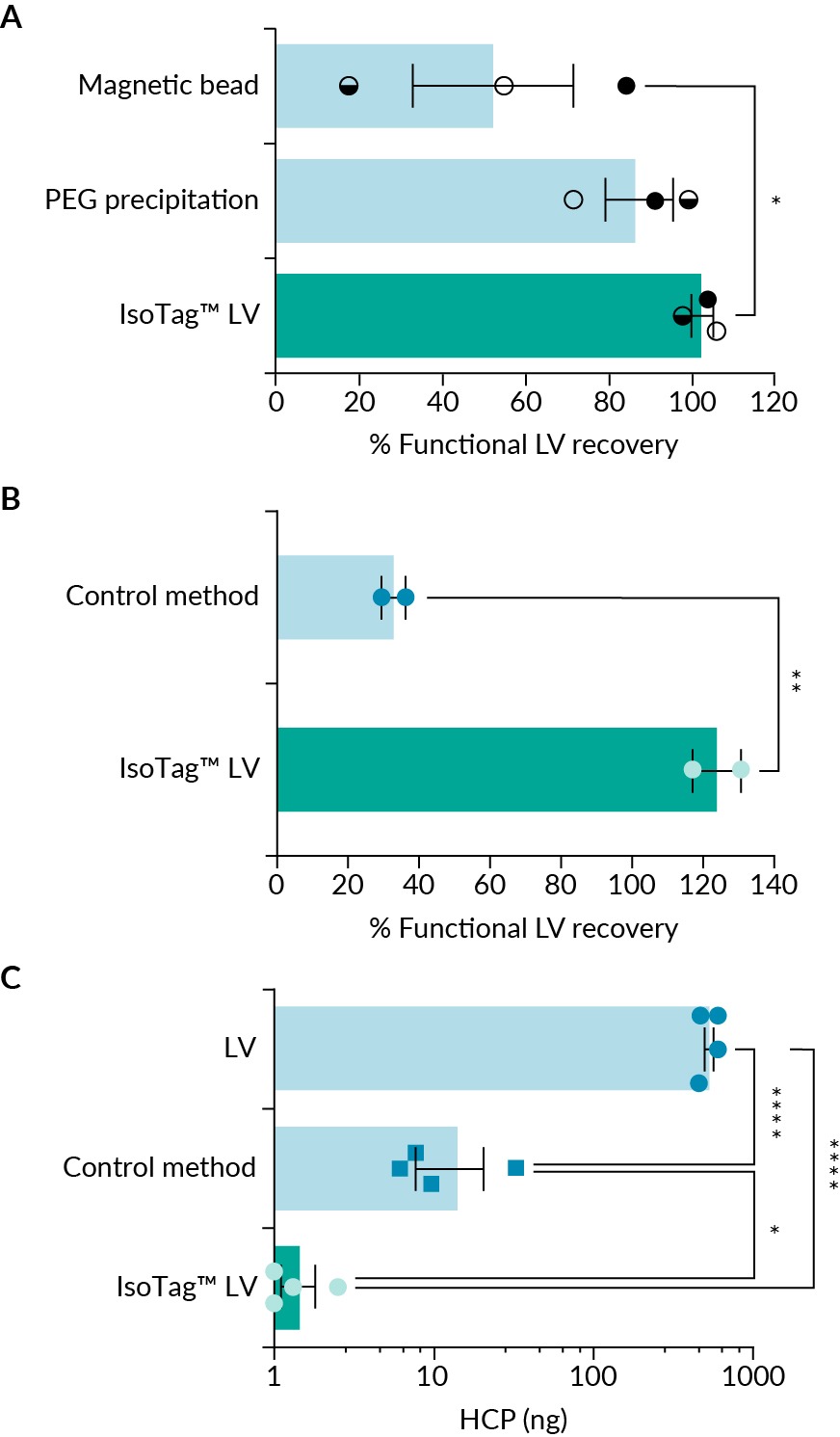 External validation using the IsoTag™ LV reagent.(A) Using high (closed circle), medium (half-filled circle), and low (open circle) starting titers, the IsoTag LV concentration reagent was compared to two competing products, PEGit™ and Mag4CLV. (B) LV recovery following concentration using IsoTag™ LV or the user’s standard PEGprecipitation method referred to here as control method. (C) Host cell protein removal using the two concentration methods compared to the starting material. Undetectable HCP samples were given a value of 1. n=2–4, error bars represent the standard error of the mean. Statistical significance calculated using a (B) students t-test or (A & C) one-way ANOVA with Fishers LSD post hoc test. *p<0.05; **p<0.01; ****p<0.0001.A & Figure S1A). These results were confirmed with an academic partner at Duke University who produces their own LV for ex vivo research use. Briefly, cells were transfected according to JetPrime protocol, [21]jetPRIME transfection reagent Short protocol. Polyplus. [22]Strash N, DeLuca S, Carattini GLJ, Heo SC, Gorsuch R, Bursac N. Human Erbb2-induced Erk activity robustly stimulates the cycling and functional remodeling of rat and human cardiomyocytes. Elife 2021; 10, e65512. and crude LV harvest material was collected, mixed, and then split equally for concentration with either their standard PEGbased overnight precipitation protocol, [22]Strash N, DeLuca S, Carattini GLJ, Heo SC, Gorsuch R, Bursac N. Human Erbb2-induced Erk activity robustly stimulates the cycling and functional remodeling of rat and human cardiomyocytes. Elife 2021; 10, e65512. [23]Lee JY, Lee HH. A new chemical complex can rapidly concentrate lentivirus and significantly enhance gene transduction. Cytotechnology 2018; 70, 193–201. or with the IsoTag™ ALPS-CF method. The research group received a paper protocol for the IsoTag™ ALPS-CF method and no additional technical support or training. Samples from both concentration methods were analyzed and compared for functional titer (via flow cytometry) and contaminant removal (via host cell protein ELISA) (Figure 6B & C & Figure S3). Comparable to data collected at Isolere, the research group was able to concentrate their crude LV 200 × with complete LV recovery using the IsoTag™ LV reagent to a titer of 1.5e10 GFU/mL (Figure 6B & Figure S3). The control method, which concentrates overnight using PEGprecipitation, recovered only 40% of the starting material when concentrated 200 ×. Concentration using IsoTag™ LV reagent was 2.5-fold more effective at recovering functional LV vectors than the control method. Furthermore, while both methods removed host cell protein contaminants from the concentrated LV product, at least 10 × more HCP was removed using the IsoTag™ LV reagent compared to the control protocol (Figure 6C). Only 1.4 ng of HCP remained in samples concentrated using the IsoTag™ LV reagent (Figure 6C), with two samples reaching undetectable levels. This is attributed to the specificity of the ALPS-CF purification process, while PEG works through non-specific precipitation by acting as a crowding agent. Of note, the IsoTag™ LV reagent did not appear to induce any cytotoxic effects in the HEK293T cells (Figure S4). Taken together, these data validate the claims made by Isolere regarding LV recovery, contaminant removal, and ease of use by the target customer of the IsoTag™ LV reagent.
External validation using the IsoTag™ LV reagent.(A) Using high (closed circle), medium (half-filled circle), and low (open circle) starting titers, the IsoTag LV concentration reagent was compared to two competing products, PEGit™ and Mag4CLV. (B) LV recovery following concentration using IsoTag™ LV or the user’s standard PEGprecipitation method referred to here as control method. (C) Host cell protein removal using the two concentration methods compared to the starting material. Undetectable HCP samples were given a value of 1. n=2–4, error bars represent the standard error of the mean. Statistical significance calculated using a (B) students t-test or (A & C) one-way ANOVA with Fishers LSD post hoc test. *p<0.05; **p<0.01; ****p<0.0001.A & Figure S1A). These results were confirmed with an academic partner at Duke University who produces their own LV for ex vivo research use. Briefly, cells were transfected according to JetPrime protocol, [21]jetPRIME transfection reagent Short protocol. Polyplus. [22]Strash N, DeLuca S, Carattini GLJ, Heo SC, Gorsuch R, Bursac N. Human Erbb2-induced Erk activity robustly stimulates the cycling and functional remodeling of rat and human cardiomyocytes. Elife 2021; 10, e65512. and crude LV harvest material was collected, mixed, and then split equally for concentration with either their standard PEGbased overnight precipitation protocol, [22]Strash N, DeLuca S, Carattini GLJ, Heo SC, Gorsuch R, Bursac N. Human Erbb2-induced Erk activity robustly stimulates the cycling and functional remodeling of rat and human cardiomyocytes. Elife 2021; 10, e65512. [23]Lee JY, Lee HH. A new chemical complex can rapidly concentrate lentivirus and significantly enhance gene transduction. Cytotechnology 2018; 70, 193–201. or with the IsoTag™ ALPS-CF method. The research group received a paper protocol for the IsoTag™ ALPS-CF method and no additional technical support or training. Samples from both concentration methods were analyzed and compared for functional titer (via flow cytometry) and contaminant removal (via host cell protein ELISA) (Figure 6B & C & Figure S3). Comparable to data collected at Isolere, the research group was able to concentrate their crude LV 200 × with complete LV recovery using the IsoTag™ LV reagent to a titer of 1.5e10 GFU/mL (Figure 6B & Figure S3). The control method, which concentrates overnight using PEGprecipitation, recovered only 40% of the starting material when concentrated 200 ×. Concentration using IsoTag™ LV reagent was 2.5-fold more effective at recovering functional LV vectors than the control method. Furthermore, while both methods removed host cell protein contaminants from the concentrated LV product, at least 10 × more HCP was removed using the IsoTag™ LV reagent compared to the control protocol (Figure 6C). Only 1.4 ng of HCP remained in samples concentrated using the IsoTag™ LV reagent (Figure 6C), with two samples reaching undetectable levels. This is attributed to the specificity of the ALPS-CF purification process, while PEG works through non-specific precipitation by acting as a crowding agent. Of note, the IsoTag™ LV reagent did not appear to induce any cytotoxic effects in the HEK293T cells (Figure S4). Taken together, these data validate the claims made by Isolere regarding LV recovery, contaminant removal, and ease of use by the target customer of the IsoTag™ LV reagent.
Discussion
The IsoTag™ technology has been described and evaluated here for its value to the field as a research-use LV purification reagent offering faster, more effective workflows that can be implemented in a high-throughput manner with simple equipment. Pure, highly concentrated LVs are of critical importance to researchers to ensure accurate and reproducible results. Furthermore, using a purification technique that can be quickly transferred to scaled-up manufacturing processes improves clinical translation of potentially lifesaving new discoveries. We sought to create a quick and easy-to-use purification reagent for researchers that repeatably and reliably concentrates LV from variable feed streams while also providing researchers with a high titer final product. LV was concentrated using the IsoTag™ LV reagent to 10 ×, 50 ×, and even 200 × with over 95% recovery with titers as high as 1e10 GFU/mL. Recoveries were consistent regardless of starting titer or the presence or absence of serum, highlighting the robustness of the IsoTag™ LV reagent. The concentrated LV product had superior contaminant removal, important for final product quality and infectivity [12]Soldi M, Sergi LS, Unali G, et al. Laboratory-scale lentiviral vector production and purification for enhanced ex vivo and in vivo genetic engineering. Mol. Ther. Methods Clin. Dev. 2020; 19, 411–425.. It is worth noting that the majority of experiments evaluating contaminant removal were performed using LV from adherent cultures, which contain fewer contaminants than LV produced from suspension cultures. Initial work has indicated similar contaminant removal profiles for LV produced from suspension cultures, but additional work remains to appropriately qualify contaminant removal with various feed streams. This will be a focus of future publications. These high recoveries and low contaminant profiles were due to the affinity-based ligand component of IsoTag™ LV reagent as well as the ALPS-CF volume-based process design. By binding only LV particles and pulling them into droplets, LV was efficiently concentrated with low-speed centrifugation regardless of starting titers or starting volumes.
Throughout the course of this evaluation, IsoTag™ LV was also found to improve LV stability and transduction. When stored at 4–8 °C, ambient temperature, and even 37 °C, LV recoveries were significantly higher in the presence of IsoTag™ LV. Reducing the sensitivity of LV to temperature variations will provide greater flexibility for researchers regarding how they process and store their LV samples. For example, a researcher can store LV at 4–8 °C for 2 weeks and have the functional titer drop only 20% compared to a titer loss of 55% when stored without IsoTag™ LV. This reduces the burden on –80 °C storage and allows the researcher easier access to their LV stocks. Additionally, protection of LV during freeze-thaw provides the user greater flexibility when using their LV stocks across multiple experiments. We hypothesize that this thermoprotection is due to IsoTag™ LV coating the LV particles, insulating them, and reducing aggregation. Further experiments will help clarify and improve upon the mechanistic understanding of this observation. The transduction enhancing properties discovered through the course of this work also warrant further exploration. We hypothesize that the IsoTag™ LV proteins could be weighing down the individual LV particles, thus bringing the particles in more direct contact with the cells, similar to spinfection [24]Berggren WT, Lutz M, Modesto V. General Spinfection Protocol. In: StemBook [internet] (Editors: Berggren WT, Lutz M, Modesto V) 2008; Cambridge (MA): Harvard Stem Cell Institute.. This phenomenon could prove quite valuable to those with low titers or hard to transduce cell lines, but work will need to be conducted in cell lines that are more difficult to transduce than HEK293T cells. Additional analysis of physical titer would also provide insight into transduction efficiency per physical particle.
Before beginning this project, a research group at Duke University was interviewed to determine their needs and interests. Final titer (and by virtue, recovery percentage) and speed were identified as two of their top priorities. Using the IsoTag™ LV reagent, they were able to concentrate their LV 200 × with virtually no loss of vector in under 4 h. Comparatively, their standard method required 18 h due to the overnight incubation and resulted in only a 40% recovery. In addition to the greater processing times required, the resulting PEGconcentrated material was highly viscous and separated during –80 °C storage. Finally, the PEGconcentrated pellet was not as compact as the IsoTag™ LVconcentrated pellet, so the researchers were unable to concentrate and purify their LV to the same extent. It has been shown that contaminant removal is an important step to produce LV vectors able to generate reproducible results [12]Soldi M, Sergi LS, Unali G, et al. Laboratory-scale lentiviral vector production and purification for enhanced ex vivo and in vivo genetic engineering. Mol. Ther. Methods Clin. Dev. 2020; 19, 411–425.. The research group at Duke University was able to remove 99% of the contaminating host cell proteins using the IsoTag™ LV reagent, a strong indicator that IsoTag™ LV was able to precisely bind the vector target using the ALPS-CF process. In summary, the IsoTag™ LV reagent effectively concentrates and purifies crude LV harvest material for research use rapidly, reliably, and robustly.
Future work on this novel reagent will include investigating the IsoTag™ LV reagent for use with other VSVG pseudotyped vectors or nanoparticles, performing studies at larger scales, and developing an efficient elution process to separate the IsoTag™ LV reagent from the highly pure LV. The IsoTag™ LV reagent binds to VSVG, so this process is not compatible with LV pseudotyped with other surface proteins but should be compatible with other VSVG containing particles. Furthermore, the mechanics of concentrating dense particles remain consistent, so larger volume processing should result in similar functional recoveries and contaminant removal. However, this remains to be tested. Last, adding an elution step to remove the IsoTag™ LV reagent will be necessary to adapt this protocol for clinical-grade LV production. While the IsoTag™ LV reagent demonstrated stabilizing properties, its toxicity in vivo has yet to be reported, although studies are ongoing.
Of note, the IsoTag™ technology is also under development for adeno-associated viral vectors and the ALPS process produces results comparable to the centrifugation-based process described herein for LV [16]Thi TTH, Pilkington EH, Nguyen DH, Lee JS, Park KD, Truong NP. The Importance of Poly(ethylene glycol) Alternatives for Overcoming PEG Immunogenicity in Drug Delivery and Bioconjugation. Polymers 2020; 12(2), 298. . We envision that the centrifugation-based concentration method outlined here (ALPS-CF) would be used for volumes up to 1L, after which it would be advantageous to use TFF to quickly concentrate and purify the droplets based on droplet size rather than density and with a filtration process that is highly scalable (ALPS-TFF). Development of the ALPS-TFF process is under development and we look forward to detailing this work in follow-on publications. The combination of ALPS-CF, described herein, with an ALPS-TFF process, would enable this platform technology to bridge a significant gap between vector material quality and purification processes utilized at different manufacturing scales and accelerate the translation of innovative medicines into clinical and commercial use.
In summary, IsoTag™ LV is a lentivirus purification reagent without compromise. It is much faster, higher yielding, and more effective than other LV purification products designed for research use because of its specificity and unique stabilizing effect on LVs. Moreover, it can be implemented by any user, at any skill level, who has access to basic lab-scale centrifugation equipment.
Materials & Methods
Cell culture
HEK293T cells (ATCC) were cultured in T75 flasks in DMEM (Cytiva Cat#: SH30243.FS) supplemented with 10% FBS (SigmaAldrich Cat#: F0926500ML), 1 × NEAA (Gibco Cat#: 11140050), 1 × penicillin/streptomycin (SigmaAldrich Cat#: P4333100ML). Cells were grown in a water jacketed incubator at 37 °C, 5% CO2, and passaged once reaching 80% confluency. Cells were discarded after reaching passage number 25.
Adherent lentivirus production
500 mL of 15e7 GFU/mL VSVG pseudotyped lentivirus harvest material with a GFP reporter was purchased from the Viral Vector Core at Duke University and was produced following the STAR protocol as published [5]Carmo M, Alves A, Rodrigues AF, et al.Stabilization of gammaretroviral and lentiviral vectors: from production to gene transfer. J. Gene Med. 2009; 11, 670–678.. In brief, the protocol outlines crude lentiviral harvest produced from adherent HEK293T cells using a calcium phosphate-based transfection. The production method recommends using a second-generation packaging system which requires three separate plasmids for transfection: one for the gene of interest, one plasmid responsible for providing necessary viral proteins, and a VSVG derived (pseudo) envelope plasmid.
LV was produced by the academic partner using the following method: when HEK293 cells were at approximately 80% confluency, media was changed (DMEM HG, 1 × Pen Strep, 10% FBS). At least 30 min after media change (up to 8 h after) cells were transfected according to the JetPrime (Polyplus Cat#101000015) protocol [21]jetPRIME transfection reagent Short protocol. Polyplus.. Briefly, plasmid DNA for PAX2 (9 µg per plate), VSVG (3 µg per plate), and the vector of interest (PRRL backbone, 18 µg per plate) were mixed with Jet Prime buffer (1000 µL per plate). Solution was vortexed for 10 sec. 50 µL of JetPrime reagent was added to the solution. Solution was vortexed for 1 sec and incubated at room temperature for 10 min. The mixture was added dropwise to each plate. 16 h after transfection, the media was changed to remove transfection reagents. Crude harvest material was collected 24–48 h after the media change. The harvested material was then spun down to remove cell debris and the supernatant was filtered with a 0.45 µm bottle top filter.
Suspension lentivirus production
Gibco™ Viral Production Cells (Thermo Scientific Cat#: A35347) cultured in a 125 mL baffled flask with 30 mL LVMAX™ Production Medium (Thermo Scientific Cat#: A3583401) passaged once reaching a density of 5 × 10⁶ cells/mL. Cells were grown in a water jacketed incubator at 37 °C, 8% CO₂, with an orbital shaker set to 125 rpm. Production followed the LVMAX™ Production System (Thermo Scientific Cat#: A35348) protocol. Cells were cultured in 250 mL baffled flasks with a starting volume of 50 mL and grown to 4.7 × 106 viable cells/mL for transfection. A total of 2.5 µg of plasmid DNA is required for every 1 mL of culture. In one conical, 1.5 µg Lentiviral packaging plasmid (Thermo Scientific Cat#: A43237) and 1 µg lentiviral transfer plasmid were diluted in OptiMEM™ I Medium (Thermo Scientific Cat#: 11058021) totaling 5% of the culture volume. In a separate conical, the LVMAX™ Transfection Reagent is also diluted to 6 mL/mL transfection culture in OptiMEM™ I Medium totaling 5% of the culture volume. The diluted plasmid DNA is added to the diluted LVMAX™ Transfection Reagent and incubated at room temperature for 10 min and then added to the culture flask immediately after the LVMAX™ Supplement (5% total volume). 5 h post transfection, the LVMAX™ Enhancer is added (4% total volume). Crude harvest material was collected 48 h post transfection. Harvest material was spun down and filtered with a 0.45 µm bottle top filter to remove cell debris.
IsoTag™ LV production
Recombinant production and purification of IsoTag™ LV reagent is comparable to the previously described production process for the IsoTag™AAV reagent [18]Haley J, Jones JB, Petraki S, et al. IsoTag™ 285 AAV: an innovative, scalable and non-chromatographic method for streamlined AAV manufacturing. Cell Gene Ther. Insights 2022; 8, 1287–1300.. 50 × stock solutions of the reagent were made using precise weights of lyophilized protein powders.
IsoTag™ LV Reagent ALPS-CF Protocol
Crude LV was mixed with 1 × IsoTag™ LV reagent and incubated on ice for 5 min. Afterward, phase transition buffer was added to 1 × final concentration, and the solution was heated to 37 °C for 5 min. The reaction mixtures were transferred to a benchtop centrifuge and centrifuged at 1000 × g for 10 min. The supernatant was removed without disturbing the pellet and saved for analysis. Cold PBS was added to the desired concentration factor, and the samples were left on rotators at 4 °C for at least 1 h until the pellet had resuspended. The academic partner performed three separate 20–80 mL 200 × concentrations using LV vectors from three independent LV productions.
Infectivity & functional titer assay
HEK293T cells were passaged into tissue culture treated 24-well plates at a density of 1 × 105 cells/mL with 0.5 mL per well. The cells were allowed to adhere overnight. The next day, lentivirus encoding GFP was added to the media at the dilution required to achieve 20–40% GFP positive cells. Cells were incubated at 37 °C, 5% CO₂ for 48 h following infection. After 48 h, with the use of the EVOS M5000 imaging system, samples were observed at 4 × with a specialty GFP (470 nm/525 nm) light cube to identify infected cells. Then, the media was replaced with Trypsin to remove the cells from the plate. Cells were washed and analyzed by flow cytometry on a BD Accuri c6 to determine the percentage of GFP-containing cells. Functional titer was measured using Green Fluorescent Units (GFU) per mL and was calculated by the following formula with the total number of cells equalling 1 × 105.
Percent recovery was calculated by dividing the calculated titer for the experimental sample by the calculated titer for the control crude harvest material and multiplying by 100.
HEK293 HCP ELISA & QuantiT™ PicoGreen™ dsDNA Assay Kit
The HEK293 HCP ELISA quantification was determined using the HEK293 HCP ELISA Kit (Cygnus Technologies Item# F650S) according to manufacturer’s instructions.
The dsDNA quantification was determined using the QuantiT™ PicoGreen™ dsDNA Assay Kit (Thermo Fisher Cat#: P7589) according to manufacturer’s instructions.
Lentivirus-associated p24 ELISA, PEGit, Mag4C, & LentiX Kits
The lentivirus associated p24 determination was done using the QuickTiter™ Lentivirus Titer Kit (Cell Biolabs Cat#: VPK107) according to manufacturer’s instructions.
The comparison products PEGit™ Virus Concentration Reagent (System Biosciences Cat#: LV810A1), Mag4CLV (Oz Biosciences Cat#: LKC11000), and LentiX Concentrator (Takara Cat#: 631232) were all tested according to manufacturer’s instructions.
Stability testing
For harvest material samples, lentivirus was diluted 1:4 in PBS with 1 × IsoTag™ LV reagent added. A control sample with diluted LV only was included. For purified virus samples, purified LV was diluted 1:100 in PBS with 1 × IsoTag™ LV added. A control sample with no IsoTag™ LV was included. For storage at 4–8 °C, a 100 µL aliquot was taken from each sample each week for functional titering. For freeze/thaw testing, 1 mL sample aliquots were frozen at –80 °C for at least 1 h. Aliquots were thawed at room temperature, and a 100 µL sample was taken for functional titer analysis. This process was repeated for an additional four freeze/thaw cycles. For ambient storage, samples were left at room temperature (22 °C± 3 °C) for 6 h and a 100 µL aliquot was taken for functional titer analysis.
Data availability statement
All data supporting the findings of this study are available within the paper and its supplemental material. Should any raw data files be needed they are available from the corresponding author upon reasonable request.
References
1. Bulcha JT, Wang Y, Ma H, et al. Viral vector platforms within the gene therapy landscape. Sig. Transduct. Target Ther. 2021; 6, 53. Crossref
2. Kumru OS, Wang Y, Gombotz CWR, et al. Physical characterization and stabilization of a lentiviral vector against adsorption and freeze-thaw. J. Pharm. Sci. 2018; 107, 2764–2774. Crossref
3. Moreira AS, Cavaco DG, Faria TQ, Alves PM, Carrondo MJT, Peixoto C. Advances in Lentivirus Purification. Biotechnol. J. 2021; 16, 2000019. Crossref
4. Rahman H, Taylor J, Clack B, Stewart RS, Canterberry SC. Effects of storage conditions on the morphology and titer of lentiviral vectors. Tex. J. Microsc. 2013; 44, 976–988. Crossref
5. Carmo M, Alves A, Rodrigues AF, et al.Stabilization of gammaretroviral and lentiviral vectors: from production to gene transfer. J. Gene Med. 2009; 11, 670–678. Crossref
6. Merten OW, Hebben M, Bovolenta C. Production of lentiviral vectors. Mol. Ther. Methods Clin. Dev. 2016; 3, 16017. Crossref
7. Valkama AJ, Oruetxebarria I, Lipponen EM, et al. Development of Large-Scale Downstream Processing for Lentiviral Vectors. Mol. Ther. Methods Clin. Dev. 2020; 17, 717–730. Crossref
8. Comisel R, Kara B, Fiesser FH; Farid SS. Lentiviral vector bioprocess economics for cell and gene therapy commercialization. Biochem. Eng. J. 2021; 167, 107868. Crossref
9. Saenz DT, Loewen N, Peretz M, et al. Unintegrated lentivirus DNA persistence and accessibility to expression in nondividing cells: analysis with class I integrase mutants. J. Virol. 2004; 78(6), 2906–2920. Crossref
10. Nguyen H, Kirkton R, Bursac N. Engineering prokaryotic channels for control of mammalian tissue excitability. Nat. Commun. 2016; 7, 13132. Crossref
11. Brown LY, Dong W . Kantor B. An improved protocol for the production of lentiviral vectors. STAR Protoc. 2020; 1(3), 100152. Crossref
12. Soldi M, Sergi LS, Unali G, et al. Laboratory-scale lentiviral vector production and purification for enhanced ex vivo and in vivo genetic engineering. Mol. Ther. Methods Clin. Dev. 2020; 19, 411–425. Crossref
13. Kutner RH, Puthli S, Marino MP, Reiser J. Simplified production and concentration of HIV-1-based lentiviral vectors using HYPERFlask vessels and anion exchange membrane chromatography. BMC Biotechnol. 2009; 9, 10. Crossref
14. PEG-it Virus Precipitation Solution. System Biosciences. Crossref
15. Liu G, Li Y, Yang L, et al. Cytotoxicity study of polyethylene glycol derivatives. RSC Adv. 2017; 7, 18252–18259. Crossref
16. Thi TTH, Pilkington EH, Nguyen DH, Lee JS, Park KD, Truong NP. The Importance of Poly(ethylene glycol) Alternatives for Overcoming PEG Immunogenicity in Drug Delivery and Bioconjugation. Polymers 2020; 12(2), 298. Crossref
17. Gaberc-Porekar Zore I, Podobnik B, Menart V. Obstacles and pitfalls in the PEGylation of therapeutic proteins. Curr. Opin. Drug Discov. Devel. 2008; 11(2), 242–250. Crossref
18. Haley J, Jones JB, Petraki S, et al. IsoTag™ 285 AAV: an innovative, scalable and non-chromatographic method for streamlined AAV manufacturing. Cell Gene Ther. Insights 2022; 8, 1287–1300. Crossref
19. Meyer D, Chilkoti A. Purification of recombinant proteins by fusion with thermally-responsive polypeptides. Nat. Biotechnol. 1999; 17, 1112–1115. Crossref
20. Yacoub N, al Romanowska M, Haritonova N, Foerster J. Optimized production and concentration of lentiviral vectors containing large inserts. J. Gene Med. 2007; 9, 579–584. Crossref
21. jetPRIME transfection reagent Short protocol. Polyplus. Crossref
22. Strash N, DeLuca S, Carattini GLJ, Heo SC, Gorsuch R, Bursac N. Human Erbb2-induced Erk activity robustly stimulates the cycling and functional remodeling of rat and human cardiomyocytes. Elife 2021; 10, e65512. Crossref
23. Lee JY, Lee HH. A new chemical complex can rapidly concentrate lentivirus and significantly enhance gene transduction. Cytotechnology 2018; 70, 193–201. Crossref
24. Berggren WT, Lutz M, Modesto V. General Spinfection Protocol. In: StemBook [internet] (Editors: Berggren WT, Lutz M, Modesto V) 2008; Cambridge (MA): Harvard Stem Cell Institute. Crossref
Affiliations
Nicole L Votaw
Isolere Bio, Inc.
Melissa Callander
Isolere Bio, Inc.
Torie Broer
Duke University Department of
Biomedical Engineering
Alyssa Wheeler
Isolere Bio, Inc.
Michael Dzuricky
Isolere Bio, Inc.
Kelli Luginbuhl
Isolere Bio, Inc.
Authorship & Conflict of Interest
Contributions: The named author takes responsibility for the integrity of the work as a whole, and has given his approval for this version to be published.
Acknowledgements: None.
Disclosure and potential conflicts of interest: Nicole L Votaw, Melissa Callander, Alyssa Wheeler, Michael Dzuricky and Kelli Luginbuhl are employees of Isolere Bio (by Donaldson). Isolere (by Donaldson) has licenses to Duke IP on materials adjacent to the inventions developed in this work. These licenses do not cover any reagents developed and presented in this work, but are not directly related. Torie Broer is an employee of Duke University. She was provided IsoTag material free of charge for performing experiments. No other compensation or resources provided. She has received funding from the National Institute of Health (National Institute of Arthritis, Musculoskeletal, and Skin Diseases).
Funding declaration: Nicole L Votaw, Melissa Callander, Alyssa Wheeler, Michael Dzuricky and Kelli Luginbuhl have received SBIR funding for research paid to Isolere.
Article & copyright information
Copyright: Published by Cell & Gene Therapy Insights under Creative Commons License Deed CC BY NC ND 4.0 which allows anyone to copy, distribute, and transmit the article provided it is properly attributed in the manner specified below. No commercial use without permission.
Attribution: Copyright © 2023 Donaldson Company, Inc. Published by Cell & Gene Therapy Insights under Creative Commons License Deed CC BY NC ND 4.0.
Article source: Invited; externally peer reviewed.
Submitted for peer review: Sep 25, 2023; Revised manuscript received: Oct 26, 2023; Publication date: Nov 1, 2023.

