Thinking ahead: developing biosynthetic blood to anticipate donor drought
Cell & Gene Therapy Insights 2022; 8(11), 1561–1570
DOI: 10.18609/cgti.2022.227
CORRIGENDUMIn the version of this article initially published, it was stated that clinical trials for Hemarina M101 had been suspended. In fact, at the time of publication, Hemarina’s HEMO2life® had just received CE approval for the preservation of kidney grafts for transplantation. The error has been corrected in the article below as of January 24 2023. The full published corrigendum can be accessed here. |
Advances in scientific research suggest the possibility of a viable biosynthetic blood technology that could radically transform the health outcomes of people around the world. Such a development would enhance a drastically insufficient global supply of red blood cells needed for transfusions, while also mitigating severe risks to donor supply precipitated by future pandemic or environmental catastrophes. However, insufficient funding is obstructing necessary further development. There is a pressing need for focused investment to advance scientific development, and an opportunity for government and philanthropic funds to help ensure that safe, efficacious biosynthetic blood becomes commercializable in a manner that enables global affordable access.
Inadequate global blood supply to meet global demand risks catastrophe
There is a tremendous global need to develop a sufficient, sustainable, and safe blood supply for a wide variety of medical needs. Blood transfusions are critical in treatments and surgeries across many disease fields, from sickle cell to cancer to renal disease and many others, as well as for addressing traumatic injuries. Hemorrhage kills approximately two million people around the world every year [1]Cannon J. Hemorrhagic Shock. N. Engl. J. Med. 2018; 378: 370–379. . It is responsible for more than a quarter of maternal deaths, approximately 70000 women, per year [2]Say L, Chou D, Gemmill A et al. Global Causes of Maternal Death: A WHO Systematic Analysis. Lancet Glob. Health 2014; 2: e323-33. . In the USA alone, approximately 40000 pints of blood are transfused daily, over 4.5 million Americans receive transfusions annually, and more than 60000 Americans die of blood loss every year [3]New York Blood Center. Blood Facts. . There are an estimated 118.5 million blood donations worldwide every year of approximately 1 pint per donation [4]World Health Organization. Blood Safety and Availability. May 26, 2022. . Unfortunately, the 118.5 million pints of blood donated annually, with the inherent challenges of maintaining a secure blood supply, are insufficient to meet global need, particularly in low- and middle-income countries (LMICs) [5]Roberts N, James S, Delaney M et al. The Global Need and Availability of Blood Products: A Modelling Study. Lancet Haematol. 2019; E606–E615. . In 2013, the World Health Organization (WHO) added whole blood and red blood cells to the Essential Medicines List, emphasizing the ‘essential’ aspect over the sticking point that blood is not a medicine per se [6]World Health Organization. The Selection and Use of Essential Medicines: Report of the WHO Expert Committee, 2013, Including the 18th WHO Model List of Essential Medicines and the 4th WHO Model List of Essential Medicines for Children. WHO Technical Report 985. 2014. .
There are stark discrepancies in access to safe blood supply between high-income countries (HICs) and LMICs. Of the 118.5 million annual blood donations worldwide, 40% are collected in HICs, and the median blood donation rate for lower-middle and low-income countries is 6.6 donations per 1000 people and 5.0 donations per 1000 people, respectively, compared to 31.5 donations per 1000 people in HICs [7]World Health Organization. Blood Safety and Availability. May 26, 2022. . A full sixty countries report less than 10 donations per 1000 people. In 2019, 61% of countries were found to have had insufficient blood supply to meet their needs, including every country in central, eastern, and western sub-Saharan Africa, Oceania, and south
Asia [5]Roberts N, James S, Delaney M et al. The Global Need and Availability of Blood Products: A Modelling Study. Lancet Haematol. 2019; E606–E615. .
This is to say nothing about the difficulties many LMICs have in maintaining or guaranteeing the safety of what little blood they may be able to access; LMICs, for a variety of reasons ranging from infrastructure to inadequate safety practices, have greater risks of transfusion-transmissible infections in the blood supply [8]Kralievits K, Raykar N, Greenberg SLM et al. The Global Blood Supply: A Literature Review. Lancet. 2015; S28: 385. . In HICs, contaminated blood transfusions – such as the disastrous transfusions by the UK’s National Health Service that led to thousands of patient deaths and many more life-threatening illnesses – are considered a national tragedy. In many LMICs, unfortunately these risks are routine, if no less devastating [9]Triggle, N. Infected Blood Victims to Get £100,000 Compensation. BBC News. August 17, 2022. .
The likelihood of future catastrophic events – whether pandemic, environmental, or both – underscores the global risks of an inadequate supply of safe blood. During the Omicron surge of the COVID-19 pandemic, a 10% reduction in blood donations prompted the American Red Cross to declare the first ever blood crisis in the USA [10]Red Cross. Red Cross Declares First-ever Blood Crisis amid Omicron Surge. January 11, 2022. , and led to unprecedented temporary hospital closures, as well as delays and cancellations of surgeries [11]Franklin J. Doctors and Patients are Facing Tough Choices Because of the National Blood Crisis. NPR. Jan 13, 2022. . The pandemic has caused decreases in donations elsewhere, as well [12]Leung JNS, Lee CK. Impact of the COVID-19 - a Regional Blood Centre’s Perspective. ISBT Science Series 2020; 1:, 362–364. . More virulent pathogens requiring extended periods of quarantine or blood-borne pathogens presenting challenges for screening blood donations may lead to an even more serious crisis of blood supply [13]Jacobs J. The Impact of Climate Change and Emerging Infectious Diseases on the Blood Supply. Transfus. Apher. Sci. 2021; 103272. . And as climate change continues to present a global threat to health and safety, extreme weather and severe weather-related disasters will further inhibit blood donations while spiking demand [14]United Nations. Climate and weather related disasters surge five-fold over 50 years, but early warnings save lives - WMO report. UN News. Sep 1, 2021. .
Biosynthetic blood offers transformative potential for global blood supply, but more development is needed
Fortunately, promising scientific research has shown success creating red blood cells (RBC) from human stem cells that mimic human donor blood, suggesting that there is a real possibility to develop an unlimited biosynthetic blood supply through creation of these biosynthetic RBCs. However, sufficient investment is needed to bridge the gap between the science and an available product. Efforts are underway to develop and manufacture biosynthetic red and white blood cells, as well as platelets using several distinct starting materials, including human pluripotent stem cells (hPSCs) – including human embryonic stem cells and human induced pluripotent stem cells – hematopoietic and progenitor stem cells, and conditionally immortalized erythroid progenitor cell lines [15]Lim ZR, Vassilev S, Leong YW et al. Industrially Compatible Transfusable iPSC-Derived RBCs: Progress, Challenges and Prospective Solutions. Int. J. Mol. Sci. 2021; 22(18): 9808.. iPSCs can be biochemically engineered and manufactured at large scale once the process is optimized, and have limitless growth and differentiation potential. In 2019, the first-in-human clinical trial took place transplanting iPSC-derived platelets into an aplastic anemia patient with alloimmune platelet transfusion refractoriness (i.e., the patient was having a negative reaction to platelets from healthy donors) [16]Sugimoto N, Kanda J, Nakamura S et al. The First-in-Human Clinical Trial of iPSC-Derived Platelets (iPLAT1): Autologous Transfusion to an Aplastic Anemia Patient with Alloimmune Platelet Transfusion Refractoriness. Blood 2021; 138(1): 351. . The clinical trial involved three transplantations and provided proof-of-concept (POC) verifying the safety of a transplanted iPSC-derived platelet product. Separately, a proof-of-concept Phase 1 trial demonstrated in-human safety and GMP manufacturing of bioengineered red blood cells, and another similar study is currently underway in the UK [17]Giarranata MC, Rouard H, Dumont A et al. Proof of principle for transfusion of in vitro–generated red blood cells. Blood 2011; 118(19): 5071–5079.. There are robust in vitro assays and tools to assess the safety and efficacy of these biosynthetic red blood cells, at least one company has indicated the ability to scale manufacturing up to 200 liters [18]Rubius Therapeutics. Rubius Therapeutics Reports First Quarter 2022 Financial Results and Provides Business Update. May 10, 2022. , and many studies exist showing the proper functioning of biosynthetic red blood cells. Recently, a collaborative research team at NHS Blood and Transplant successfully expanded isolated hematopoietic stem cells from adult peripheral blood by 100,000-fold, in which, 30% is transfusable post filtration [19]Gallagher J. Lab-grown blood given to people in world-first clinical trial. BBC News. Nov 7, 2022. . Among the ten healthy volunteers in this clinical trial, two have begun normal and biosynthetic blood transfusions to study its intended efficacy.
Unlike artificial blood, which encompasses molecules and polymers that mimic the oxygen carrying capacity of red blood cells but has a history of side effects and short lifetime when transfused into the body, biosynthetic blood has a more promising chance of functioning as an effective replacement for donor blood (see Figure 1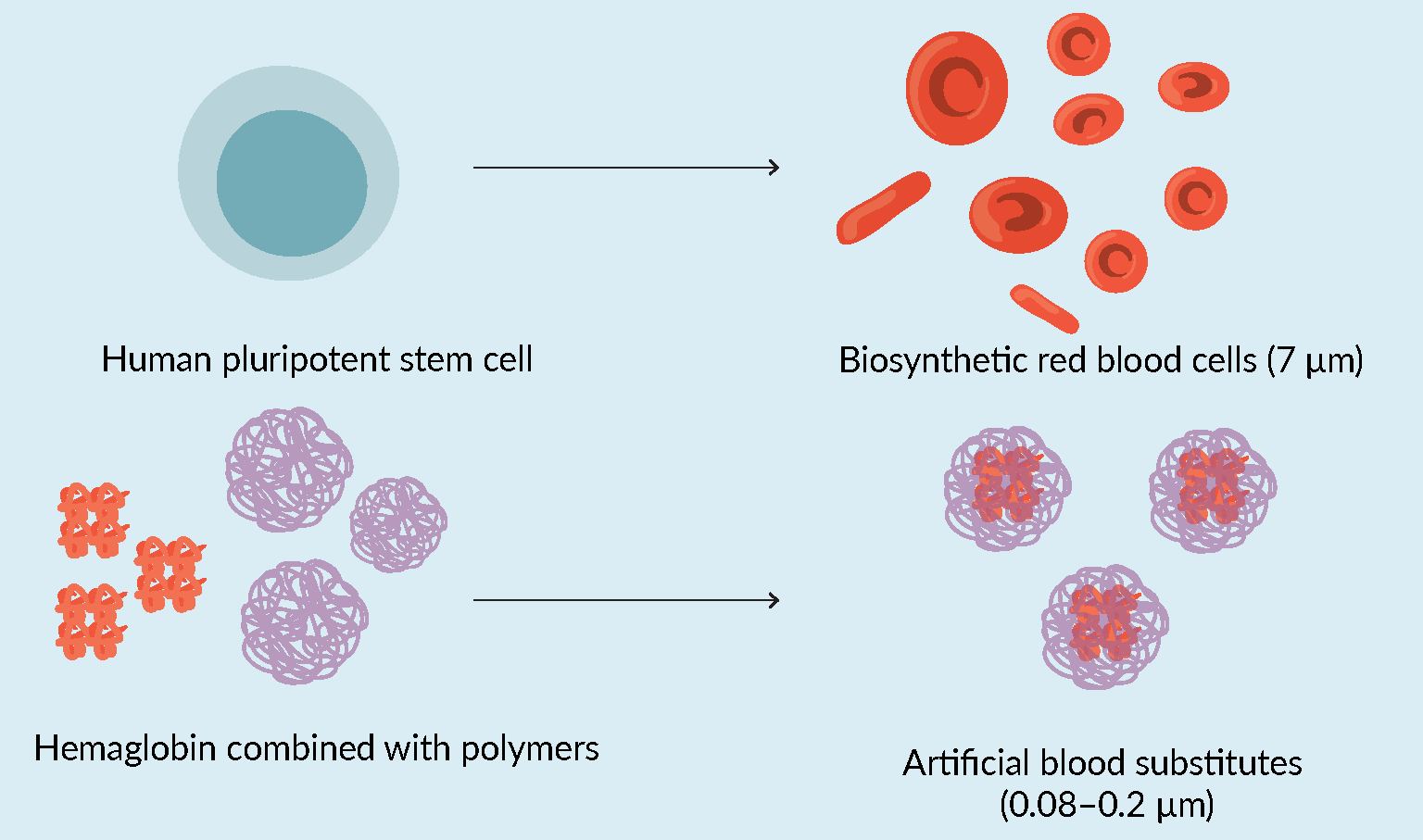 Definition of biosynthetic versus artificial blood research.).
Definition of biosynthetic versus artificial blood research.).
A safe, efficacious, and non-limited supply of biosynthetic blood would be a game-changer – alleviating the strain on an otherwise unsustainable global blood supply by reducing overreliance on the availability of donations, and greatly reducing the risk of transmissions of pathogens via transfusion. For LMICs in particular, biosynthetic blood offers the potential of building up supply for critical services. For the nearly one-third of women in sub-Saharan Africa and South Asia who are anemic and thus at elevated risk of postpartum hemorrhage, biosynthetic blood may make the difference between life and death [20]Stevens, G, Finucane M, De-Regil LM et al. Global, regional, and national trends in haemoglobin concentration and prevalence of total and severe anaemia in children and pregnant and non-pregnant women for 1995–2011: a systematic analysis of population-representative data. Lancet Glob. Health 2013; 1(1): E16–E25. [21]Daru, J, Zamora J, Fernández-Félix BM et al. Risk of maternal mortality in women with severe anaemia during pregnancy and post partum: a multilevel analysis. Lancet Glob. Health 2018; 6(5), E548–554. . For the many patients in LMICs who need transfusions for any of a wide variety of illnesses, including children who have high prevalences of severe anemia and the 236000 children in Sub-Saharan Africa born each year with sickle-cell disease [20]Stevens, G, Finucane M, De-Regil LM et al. Global, regional, and national trends in haemoglobin concentration and prevalence of total and severe anaemia in children and pregnant and non-pregnant women for 1995–2011: a systematic analysis of population-representative data. Lancet Glob. Health 2013; 1(1): E16–E25. [22]Zhou, A, Travassos, M. Bringing Sickle-Cell Treatments to Children in Sub-Saharan Africa. N. Engl. J. Med. 2022; 387: 488–491. , access to biosynthetic blood may lead to improved health outcomes.
Unfortunately, further development has been stifled by insufficient funding. Federal agencies have focused funding for decades on artificial (synthetic) blood substitutes based on purified forms of hemoglobin to provide oxygen carrying capacity in acute blood loss situations [23]. Despite some advances, no synthetic blood substitutes are approved for use in the US or Europe. Significant safety issues and high mortality [24], including myocardial infarction and death, have been associated with the use of these artificial blood products [24]. Some synthetic products such as Erythromer [25] are still proceeding through early-stage development. In a first for the category of oxygen carriers Hemarina’s HEMO2life® just received CE approval for preservation of kidney grafts for transplantation [26]. And while a retrospective study of ten patients showed one artificial blood product to provide an effective oxygen bridge for patients unable to be transfused with RBC [27], other case reports illustrate the significant challenges with use of artificial blood [28].
Funding from government, foundations and investors for creation of new therapies from stem cell science has been heavily weighted on the development and commercialization of white blood cells to be used for cancer therapies (e.g., CAR-T cells for use against cancer). The price of a bag of blood or platelets is a tiny fraction of the price of a cancer therapy, making it a less attractive investment opportunity for the medical industry. Lack of funding from investors and industry has hindered the development of biosynthetic blood products able to address urgent, life-threatening issues globally.
Government & philanthropic investment should push the development of biosynthetic blood to ensure sustainable, commercially viable & equitable global access
The current status of the research leaves a significant opportunity for increased government and philanthropic funding to catalyze the development of biosynthetic red blood cells, including efficient and effective manufacturing processes that will ensure worldwide availability in ways that are attuned to infrastructure limitations in LMICs. The Defense Advanced Research Projects Agency (DARPA), with its ‘Blood Pharming’ program, has demonstrated some interest in this field as well due to logical applications for the military [29]Defense Advanced Research Projects Agency. Pursuit of Scalable, On-Demand Blood for Transfusions Could Yield Novel Means of Therapeutics Delivery. Nov 12, 2013. . The Biomedical Advanced Research and Development Authority (BARDA) has provided some funding for the development of human stem-cell derived platelets [30] Platelet BioGenesis Receives Contract Worth Up to $56 Million from the Biomedical Advanced Research and Development Authority (BARDA) to Develop Human Stem Cell-Derived Platelets as a Medical Countermeasure to Radiological and Nuclear Exposure. Business Wire. Oct 7, 2019.. These are positive advances, but more targeted investment is needed in a manner that fosters increased collaboration while appropriately reflecting urgency. Funding to date has helped academic labs to do preliminary research, but there has not been a unified effort to raise funds to get biosynthetic blood from concept to patient. Such an effort would constitute a major investment in pandemic and catastrophe preparedness that, as with the important research done on mRNA vaccines prior to the COVID-19 pandemic, may help mitigate future crises.
Translating biosynthetic blood from concept to patient will require targeted funding to advance efforts in (1) generating functionally mature RBCs and (2) scaling up RBC manufacturing to enable cost-effective high-volume production of therapeutic dosages.
For instance, human pluripotent stem cells are a favored starting material for RBC generation [15]Lim ZR, Vassilev S, Leong YW et al. Industrially Compatible Transfusable iPSC-Derived RBCs: Progress, Challenges and Prospective Solutions. Int. J. Mol. Sci. 2021; 22(18): 9808.. However, hiPSC-derived RBCs remain functionally immature; they express fetal globins (HbF) as opposed to adult globins (HbA). Fetal and adult hemoglobins have different oxygen binding kinetics, meaning that this difference in protein expression affects cell functionality and therefore potential clinical efficacy [31]Sivalingam, J, SuE Y, Lim ZR et al. A Scalable Suspension Platform for Generating High-Density Cultures of Universal Red Blood Cells from Human Induced Pluripotent Stem Cells. Stem Cell Reports 2021; 16(1), 182–197. . While there are efforts to both improve the maturity of hiPSC-derived RBCs and assess the clinical potential of the immature hiPSC-derived RBCs [32]Yang, C-T, Ma R, Axton RA et al. Activation of KLF1 Enhances the Differentiation and Maturation of Red Blood Cells from Human Pluripotent Stem Cells. Stem Cells 2017; 35(4), 886–897. [33]Kobari, L, Yates F, Oudrhiri N et al. Human induced pluripotent stem cells can reach complete terminal maturation: in vivo and in vitro evidence in the erythropoietic differentiation model. Haematologica 2012; 97(12): 1795–1803. it is clear that additional effort is needed to clear this scientific hurdle.
In addition to improvements in cell differentiation protocols, advancements in biomanufacturing will be necessary to enable cost-effective biosynthetic RBC production at the scale necessary to generate therapeutic doses (one unit of blood = 2 trillion RBCs). Currently, hospitals in the USA use roughly 29,000 units of RBCs per day. This represents an annual requirement to manufacture
>2×1019 cells. Generously assuming upstream cell densities of 5×108 cells/ml are achievable and a total process cycle time of 21-days, approximately 2.4 million liters in biomanufacturing capacity (i.e., cumulative bioreactor volume), producing 42.3 million liters of upstream product, would be required to supply the US market alone. For context, as of 2021, the total US biomanufacturing capacity was 5.5 million liters distributed across 598 facilities. Biomanufacturing on this scale for a single product is unprecedented and presents a range of complex logistical and engineering challenges.
Practically, the first use of biosynthetic RBCs would be for niche transfusion applications because of lower production volume requirements and higher sale prices. Hospitals typically pay between $200–300 USD per unit of RBCs ($0.10–0.15 per billion RBCs). As RBCs are a high-volume commodity used across a wide variety of applications, the average value-add for a unit of biosynthetic RBCs is not meaningfully higher than a donated unit. Accordingly, assuming profitability could be achieved with a 25% gross margin, the COGs target for a unit of biosynthetic RBCs becomes $150–225 USD ($0.08–0.11 per billion RBCs). The targeted application of biosynthetic RBCs in applications particularly underserved by donor derived RBC products has the potential to enable market entry at a higher sale price. For example, patents alloimmunization to RBC antigens must be transfused with phenotypically matched units of RBC, which are more expensive to procure. Biosynthetic RBCs with a broadly compatible phenotype would be a suitable replacement for the more expensive phenotypically matched units. However, even within niche cases, it is unlikely that the price premium would be both significant and cover a large enough patient population to recover commercialization costs.
To achieve COGs of $0.08–0.11 per billion RBCs it is likely that every inefficiency associated with the source of cells, upstream manufacturing, downstream processing, and reagent costs must be overcome. As mentioned previously, multiple cell sources have been used successfully to generate biosynthetic RBCs. Among them, self-renewing sources, such as iPSCs or conditionally immortalized erythroid cell lines, are preferred over primary stem cells as primary stem cells are challenging to procure in large quantities and they introduce variability into the manufacturing process. Currently no viable self-renewing source of starting material that is genetically stable, produces adult hemoglobin, and has a high enucleation rate has been developed.
The primary challenges in upstream manufacturing are achievable cell densities and scale-up. Due to the massive quantity of cells in each therapeutic dose of RBCs, ultra-high cell densities are required to mitigate unreasonable liquid handling requirements. In order to achieve and support these densities, media intensive perfusion cell culture strategies are likely to be needed. Scaling-up the process into large volume bioreactors will be essential to achieve commercially relevant production volumes. The ideal upstream process, with lowest potential variable costs, would be fully automated and operate continuously for several months. However, bioreactor technology optimized for continuous manufacturing of biosynthetic RBCs would need to be developed.
The enucleation rate and downstream efficiency must be improved substantially. The largest efficiency loss for self-renewing cell sources is the rate at which they eject their nuclei and transform from late-stage erythroid progenitors into reticulocytes. Ideally, this rate would be above 90% and comparable to those achieved with primary stem cells. Furthermore, downstream processes that can efficiently process large volumes while purifying enucleated cells from closely related nuclei and nucleated cells must be developed and optimized.
Expensive reagents are also noted as a high cost in RBC biomanufacturing [15]Lim ZR, Vassilev S, Leong YW et al. Industrially Compatible Transfusable iPSC-Derived RBCs: Progress, Challenges and Prospective Solutions. Int. J. Mol. Sci. 2021; 22(18): 9808.; the high cost of reagents per cell could be reduced either through the development of high-density processes or the systematic reduction of reagent component costs [34]Kuo H-H, Gao X, DeKeyser JM et al. Negligible-Cost and Weekend-Free Chemically Defined Human iPSC Culture. Stem Cell Rep. 2020; 14(2): 256–270.. Other challenges that must be overcome to achieve cost-effective scalable manufacturing are apparent in other realms of cell therapy manufacturing as well. For instance, high facility operation costs could be reduced by the development of closed systems that enable use of lower-grade cleanrooms [35]Lipsitz, Y, Milligan W, Fitzpatrick I et al. A Roadmap for Cost of Goods Planning to Guide Economic Production of Cell Therapy Products. Cytotherapy 2017; 19(12): 1383–1391. ; high labor costs could be reduced by the development of automated processes [36]Smith D, Heathman TRJ, Klarer A et al. Towards Automated Manufacturing for Cell Therapies. Curr. Hematol. Malig. Rep. 2019; 14: 278–285..
Philanthropy typically plays a comparatively smaller role in the medical research ecosystem, but can fill key funding gaps to de-risk science and shine a light on overlooked yet impactful projects, and could play a critical part here. Philanthropic dollars banded together for recurring investment may help bring the technology past proof of concept to an inflection point.
With biosynthetic blood, additional focused funding from these sources could help move the science through the high costs of translational research and ensure that a commercializable product can be manufactured cost-effectively and at a scale sufficient to create an attractive market opportunity, aligning critical investment criteria with clear societal demand. The lack of adequate industry funding in biosynthetic blood development also presents an opportunity for government and non-government investors to link the funding with clear commitments related to health equity and global equitable access [37]Goldman AS, Ramachandran R. How The Next NIH Director Can Ensure Global Equitable Access To Medical Technologies. Health Affairs Forefront. Aug 25, 2022. , in acknowledgment of the dire needs in LMICs and the need to have biosynthetic blood be made available and affordable.
Biographies
Andrew Spencer Goldman is general counsel and head of policy at Roivant Social Ventures (RSV), a 501c3 social impact organization working to make systemic improvements to health equity. Prior to RSV, he worked with the Medicines Patent Pool (MPP) in Geneva, Switzerland, where he negotiated and drafted public health-driven patent and know-how licenses to increase affordable access for treatments and other medical technologies for COVID-19, HIV, HCV, and tuberculosis. Previously, Goldman was counsel for policy and legal affairs at Knowledge Ecology International (KEI) where he worked on global access to medicines issues. Goldman received a BA from Princeton University, an MA from Columbia University, and his JD from the University of Maryland School of Law and maintains bar memberships in New York and Maryland.
Shane Kilpatrick is a co-founder of Membio Inc., a venture-backed biotechnology company enabling the future of medicine by making cells easier to grow. He received his MASc in chemical engineering and Master of Business, Entrepreneurship and Technology from the University of Waterloo, where he founded Membio and began the development of their proprietary biomanufacturing technology. He has led Membio into world leading accelerator programs and raised over $ 4 million in equity financing their continued development. He has several pending patents and academic research papers and was the recipient of the Norman Esch entrepreneurship award.
Marinna Madrid is the Chief Product Officer and Co-Founder at Cellino, a venture capital-backed biotech company building the next generation of cell-based tissues and therapies with a proprietary platform technology. She received her PhD and MA in Applied Physics from Harvard University, where she co-invented laser-based intracellular delivery techniques. She received her BSc in Biophysics from University of California, Los Angeles, after transferring from Riverside Community College. She is the recipient of the Harvard Graduate Prize Fellowship, the Catalyst Accelerator Grant from Harvard Medical School, and is on the Forbes 30 Under 30 2019 list for Healthcare. She has several patents, peer-reviewed publications, and wrote the first review paper on autologous iPSC-based cell therapies.
Zhong Ri Lim is a Staff Research Officer in Bioprocessing Technology Institute, A*STAR. Over the past six years of research in human adult and pluripotent stem cell bioprocessing, he focused primarily on red blood cells. He has published several papers in which he utilized a multifactorial Design of Experiment (DoE) approach for allowing cost reduction in immortalized erythroblast expansion and a scalable platform for high density cultures of hiPSC-derived erythroblasts. His most recent work is on improving erythroid maturation efficacy for hiPSC-derived erythroblasts.
Steve Oh’s research has been on human adult and pluripotent stem cell bioprocessing. His team has developed a variety of patent families for the manufacture of mesenchymal stem cells, reprogrammed human induced pluripotent stem cells and created neural cells, cardiomyocytes, blood cells, cartilage, bone and retinal pigment epithelial cells at bioreactor scale using a range of microcarrier technologies including biodegradable ones. Professor Oh and team have developed biodegradable microcarriers for cell manufacturing and microfluidics devices for microcarrier, cell and debris separation. Most recently, Professor Oh has achieved a novel method of directed differentiation using CRISPR technology that will accelerate therapeutic applications of stem cells, and has also created a machine learning model for predicting cartilage repair with MSC. Steve has received research funding grants totaling SGD 25 million. He has 43 Patents, granted and pending, over 135 scientific publications, written 2 books and created 3 companies.
Taylor Rose contributed to this article while studying for her MBA from the Wharton School at the University of Pennsylvania. She was an early member of the Roivant Social Ventures team as the first Global Access Fellow. Previously, she worked as the manager of the Children’s Hospital of Philadelphia (CHOP)’s CURED group, the nation’s first clinic dedicated to delivering and developing curative therapies for sickle cell disease and other red blood cell disorders. Taylor is currently a management consultant based in New York and has a SB from MIT.
Lena Patel is a senior associate at Roivant Sciences, where she reports to the Chairman of the Board of the companies in the Roivant Pharma family to support the strategic and financial management of those businesses. Prior to Roivant, she spent over two years in Healthcare Investment Banking at the Royal Bank of Canada performing services on all aspects of merger and acquisition transactions, as well as debt and equity financings within the biotech, pharma and drug contract manufacturing verticals. She received her bachelors in Biology from Duke University where she did neurology research under the mentorship of Dr Simon Davis at the Alzheimer’s Disease Research Center.
Barbara Nelsen is the founder of Nelsen Biomedical (NBM), a boutique life sciences strategy consulting firm with offices in Minneapolis and Boston. NBM has partnered with over forty firms from start-ups (e.g., Rebiotix, acquired by Ferring Pharmaceuticals) to global conglomerates (e.g., Kawasaki Heavy Industries) and brings strong expertise in regenerative medicine and cell therapy manufacturing. Dr Nelsen brings over 20 years experience working in R&D, business and strategic development within the biotechnology and pharmaceutical industries. She currently serves on the boards of: Sanaby Health, a special purpose acquisition company; Regenerative Medicine Minnesota, a state initiative to fund research and business growth in regenerative medicine; Anatomic Corporation, an iPSC-based tools company; and University Enterprise Labs, a start-up accelerator for life science and healthcare companies. Dr Nelsen holds a PhD in molecular biology and an MBA.
References
1. Cannon J. Hemorrhagic Shock. N. Engl. J. Med. 2018; 378: 370–379. Crossref
2. Say L, Chou D, Gemmill A et al. Global Causes of Maternal Death: A WHO Systematic Analysis. Lancet Glob. Health 2014; 2: e323-33. Crossref
3. New York Blood Center. Blood Facts. Crossref
4. World Health Organization. Blood Safety and Availability. May 26, 2022. Crossref
5. Roberts N, James S, Delaney M et al. The Global Need and Availability of Blood Products: A Modelling Study. Lancet Haematol. 2019; E606–E615. Crossref
6. World Health Organization. The Selection and Use of Essential Medicines: Report of the WHO Expert Committee, 2013, Including the 18th WHO Model List of Essential Medicines and the 4th WHO Model List of Essential Medicines for Children. WHO Technical Report 985. 2014. Crossref
7. World Health Organization. Blood Safety and Availability. May 26, 2022. Crossref
8. Kralievits K, Raykar N, Greenberg SLM et al. The Global Blood Supply: A Literature Review. Lancet. 2015; S28: 385. Crossref
9. Triggle, N. Infected Blood Victims to Get £100,000 Compensation. BBC News. August 17, 2022. Crossref
10. Red Cross. Red Cross Declares First-ever Blood Crisis amid Omicron Surge. January 11, 2022. Crossref
11. Franklin J. Doctors and Patients are Facing Tough Choices Because of the National Blood Crisis. NPR. Jan 13, 2022. Crossref
12. Leung JNS, Lee CK. Impact of the COVID-19 - a Regional Blood Centre’s Perspective. ISBT Science Series 2020; 1:, 362–364. Crossref
13. Jacobs J. The Impact of Climate Change and Emerging Infectious Diseases on the Blood Supply. Transfus. Apher. Sci. 2021; 103272. Crossref
14. United Nations. Climate and weather related disasters surge five-fold over 50 years, but early warnings save lives - WMO report. UN News. Sep 1, 2021. Crossref
15. Lim ZR, Vassilev S, Leong YW et al. Industrially Compatible Transfusable iPSC-Derived RBCs: Progress, Challenges and Prospective Solutions. Int. J. Mol. Sci. 2021; 22(18): 9808. Crossref
16. Sugimoto N, Kanda J, Nakamura S et al. The First-in-Human Clinical Trial of iPSC-Derived Platelets (iPLAT1): Autologous Transfusion to an Aplastic Anemia Patient with Alloimmune Platelet Transfusion Refractoriness. Blood 2021; 138(1): 351. Crossref
17. Giarranata MC, Rouard H, Dumont A et al. Proof of principle for transfusion of in vitro–generated red blood cells. Blood 2011; 118(19): 5071–5079. Crossref
18. Rubius Therapeutics. Rubius Therapeutics Reports First Quarter 2022 Financial Results and Provides Business Update. May 10, 2022. Crossref
19. Gallagher J. Lab-grown blood given to people in world-first clinical trial. BBC News. Nov 7, 2022. Crossref
20. Stevens, G, Finucane M, De-Regil LM et al. Global, regional, and national trends in haemoglobin concentration and prevalence of total and severe anaemia in children and pregnant and non-pregnant women for 1995–2011: a systematic analysis of population-representative data. Lancet Glob. Health 2013; 1(1): E16–E25. Crossref
21. Daru, J, Zamora J, Fernández-Félix BM et al. Risk of maternal mortality in women with severe anaemia during pregnancy and post partum: a multilevel analysis. Lancet Glob. Health 2018; 6(5), E548–554. Crossref
22. Zhou, A, Travassos, M. Bringing Sickle-Cell Treatments to Children in Sub-Saharan Africa. N. Engl. J. Med. 2022; 387: 488–491. Crossref
23. Chen J-Y, Scerbo M, Kramer G. A Review of Blood Substitutes: Examining The History, Clinical Trial Results, and Ethics of Hemoglobin-Based Oxygen Carriers. Clinics. 2009; 64(8): 803–13. Crossref
24. Natanson, C, Kern S, Lure P et al. Cell-free hemoglobin-based blood substitutes and risk of myocardial infarction and death: a meta-analysis. JAMA 2008; 299(19): 2304–12. Crossref
25. When is a red blood cell substitute needed? KaloCyte. Crossref
27. Zumberg, M, Gorlin J, Griffiths A et al. A case study of 10 patients administered HBOC-201 in high doses over a prolonged period: outcomes during severe anemia when transfusion is not an option. Transfusion 2020; 60(5): 932–939. Crossref
28. Barrett, C, Theodore S, Dechert T et al. Resuscitation of an exsanguinated obstetrics patient with HBOC-201: A case report. Transfusion. 2022; 62(Suppl. 1): S218–S223. Crossref
29. Defense Advanced Research Projects Agency. Pursuit of Scalable, On-Demand Blood for Transfusions Could Yield Novel Means of Therapeutics Delivery. Nov 12, 2013. Crossref
30. Platelet BioGenesis Receives Contract Worth Up to $56 Million from the Biomedical Advanced Research and Development Authority (BARDA) to Develop Human Stem Cell-Derived Platelets as a Medical Countermeasure to Radiological and Nuclear Exposure. Business Wire. Oct 7, 2019. Crossref
31. Sivalingam, J, SuE Y, Lim ZR et al. A Scalable Suspension Platform for Generating High-Density Cultures of Universal Red Blood Cells from Human Induced Pluripotent Stem Cells. Stem Cell Reports 2021; 16(1), 182–197. Crossref
32. Yang, C-T, Ma R, Axton RA et al. Activation of KLF1 Enhances the Differentiation and Maturation of Red Blood Cells from Human Pluripotent Stem Cells. Stem Cells 2017; 35(4), 886–897. Crossref
33. Kobari, L, Yates F, Oudrhiri N et al. Human induced pluripotent stem cells can reach complete terminal maturation: in vivo and in vitro evidence in the erythropoietic differentiation model. Haematologica 2012; 97(12): 1795–1803. Crossref
34. Kuo H-H, Gao X, DeKeyser JM et al. Negligible-Cost and Weekend-Free Chemically Defined Human iPSC Culture. Stem Cell Rep. 2020; 14(2): 256–270. Crossref
35. Lipsitz, Y, Milligan W, Fitzpatrick I et al. A Roadmap for Cost of Goods Planning to Guide Economic Production of Cell Therapy Products. Cytotherapy 2017; 19(12): 1383–1391. Crossref
36. Smith D, Heathman TRJ, Klarer A et al. Towards Automated Manufacturing for Cell Therapies. Curr. Hematol. Malig. Rep. 2019; 14: 278–285. Crossref
37. Goldman AS, Ramachandran R. How The Next NIH Director Can Ensure Global Equitable Access To Medical Technologies. Health Affairs Forefront. Aug 25, 2022. Crossref
Affiliations
Andrew S Goldman, JD, MA
General Counsel and Head of Policy,
Roivant Social Ventures
Shane Kilpatrick, MASc, MBET
Co-founder,
Membio Inc.
Marinna Madrid, PhD
Chief Product Officer and Co-Founder,
Cellino
Zhong Ri Lim
Staff Research Officer in Bioprocessing Technology Institute,
A*STAR
Steve Oh, PhD
Director of the Stem Cell Bioprocessing Group,
A*STAR
Taylor Rose, MBA
Management Consultant
Lena Patel
Senior Associate,
Roivant Sciences
Barbara A Nelsen, PhD, MBA
Founder,
Nelsen Biomedical
Authorship & Conflict of Interest
Contributions: All named authors take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Acknowledgements: None.
Disclosure and potential conflicts of interest: Kilpatrick S discloses he has patents pertaining to novel bioreactor technology suitable for cost-effective large-scale manufacturing of cells, has a leadership role as CEO and has large ownership stake in Membio Inc. Madrid M discloses she is an employee and shareholder at Cellino Biotech, Inc. Patel L discloses she has stocks through Roivant employee equity issued as part of salary. The other authors have no conflicts of interest to disclose.
Funding declaration: Kilpatrick S received financial support for the research, authorship and/or publication of this article from Membio Inc. Patel L received financial support for the research, authorship and/or publication of this article as she volunteers through Roivant Social Ventures, which is affiliated with Roivant Sciences.
Article & copyright information
Copyright: Published by Cell and Gene Therapy Insights under Creative Commons License Deed CC BY NC ND 4.0 which allows anyone to copy, distribute, and transmit the article provided it is properly attributed in the manner specified below. No commercial use without permission.
Attribution: Copyright © 2022 Nelsen Biomedical. Published by Cell and Gene Therapy Insights under Creative Commons License Deed CC BY NC ND 4.0.
Article source: Invited.
Revised manuscript received: Dec 7 2022; Publication date: Jan 3 2023; Article corrected: Jan 24 2023.
