Roadmap to success in AAV purification. In-process control, high throughput & novel column modalities as necessary means for control over scalable AAV process
Cell & Gene Therapy Insights 2022; 8(10), 1315–1328
DOI: 10.18609/cgti.2022.192
Manufacture and purification of recombinant adeno-associated viruses (rAAV) require development and optimization of processes to ensure the best possible quality of the final rAAV product. To do so, different strategies in upstream can be used to achieve the highest possible viral titer and lowest amount of impurities, both of which further influence downstream. Second challenge involves removal of cell debris where different pre-treatments can be utilized. In the next step, optimized capture of rAAV on a cation-exchange chromatography should be developed to remove impurities and achieve a high recovery of rAAV. In the end, several chromatographic options are available to remove empty and defected capsids, so only functional viruses can be isolated. Here, the process of manufacturing and purification of rAAV has been designed using monolithic columns to achieve this important goal of preparing rAAV for the use in gene therapy.
Purification of rAAV using chromatographic columns is one of the key purification process solutions in gene therapy industry. Common rAAV industrial manufacturing platform is based on two chromatographic steps: capture and polishing. During capture step, rAAV is bound, concentrated, and partially purified using cation exchange or affinity chromatography. In the next step, rAAV is further purified and enriched using anion exchange column or ultracentrifugation. The overall bioprocess yield and purity of the final product depend not only on downstream steps, but on upstream process as well, since certain harvest attributes can largely affect column capacity, and consequentially downstream process efficiency. This is partially addressed utilizing different pre-treatment strategies designed to simplify and increase productivity of early downstream steps, but there is no universal solution for highly variable impurity profile during rAAV production in cells. Manufacturing process ends with buffer exchange to formulation buffer, followed by fill and finish (Figure 1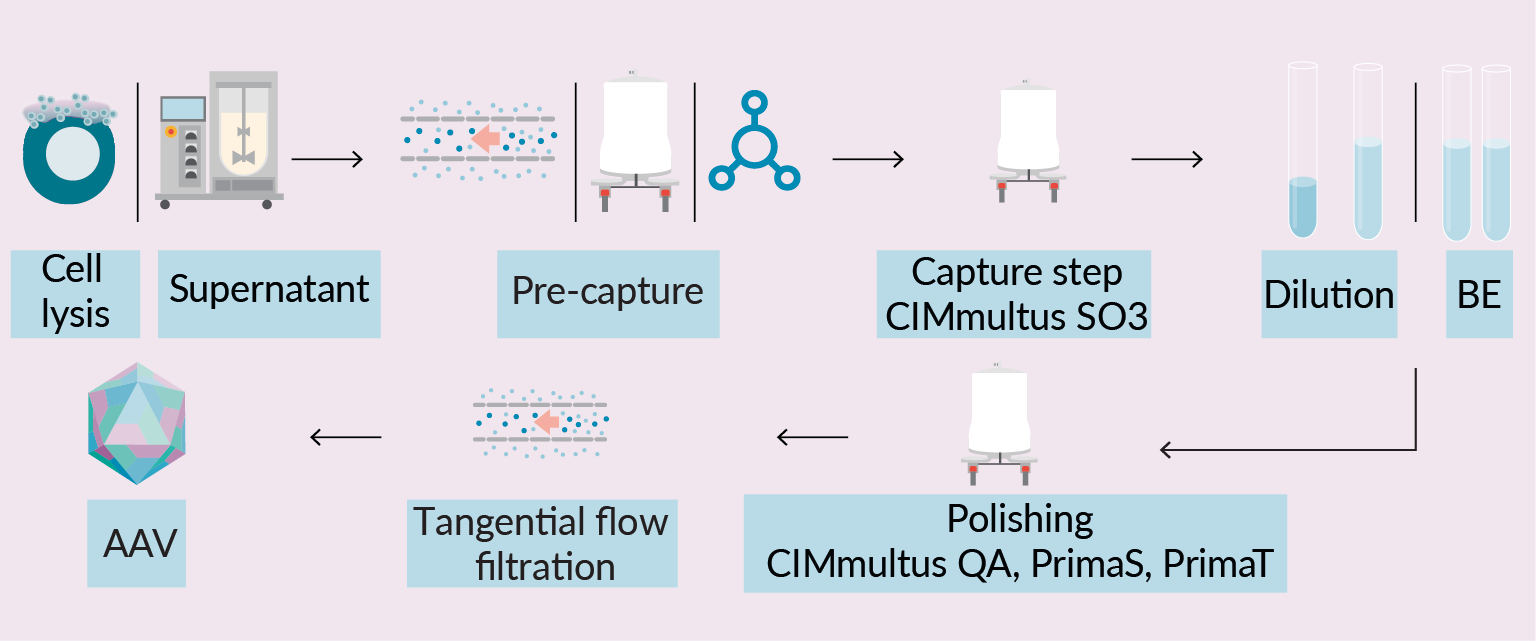 Schematic representation of downstream processing of complex rAAV samples using monolithic columns.BE: Buffer exchange; TFF: Tangential flow filtration; AAV: Adeno-associated virus.) [1]Penaud-Budloo M, François A, Clément N, Ayuso E. Pharmacology of Recombinant Adeno-associated Virus Production. Mol. Ther. Methods Clin. Dev. 2018; 8, 166–180. [2]Gagnon P, Leskovec M, Goricar B, Štrancar A. Streamlining Industrial Purification of Adeno-Associated Virus. Bioprocess. Int. 2020; 18(11–12), 1–4. [3]Qu W, Wang M, Wu Y, Xu R. Scalable Downstream Strategies for Purification of Recombinant Adeno- Associated Virus Vectors in Light of the Properties. Curr. Pharm. Biotechnol. 2015; 16(8), 684–695. [4]Potter M Lins B, Mietzsch M et al. A simplified purification protocol for recombinant adeno-associated virus vectors. Mol. Ther. Methods Clin. Dev. 2014; 1, 14034..
Schematic representation of downstream processing of complex rAAV samples using monolithic columns.BE: Buffer exchange; TFF: Tangential flow filtration; AAV: Adeno-associated virus.) [1]Penaud-Budloo M, François A, Clément N, Ayuso E. Pharmacology of Recombinant Adeno-associated Virus Production. Mol. Ther. Methods Clin. Dev. 2018; 8, 166–180. [2]Gagnon P, Leskovec M, Goricar B, Štrancar A. Streamlining Industrial Purification of Adeno-Associated Virus. Bioprocess. Int. 2020; 18(11–12), 1–4. [3]Qu W, Wang M, Wu Y, Xu R. Scalable Downstream Strategies for Purification of Recombinant Adeno- Associated Virus Vectors in Light of the Properties. Curr. Pharm. Biotechnol. 2015; 16(8), 684–695. [4]Potter M Lins B, Mietzsch M et al. A simplified purification protocol for recombinant adeno-associated virus vectors. Mol. Ther. Methods Clin. Dev. 2014; 1, 14034..
Therapeutic applications of rAAV-based gene therapy vectors require removal of process and product related impurities, as they represent serious safety threats. Their removal burdens the economics of the manufacturing. The most critical subset of process-related impurities are host cell impurities (hcDNA, hc proteins and chromatin complexes) as well as plasmids and transfection reagents used for rAAV production. On the other hand, the most critical subsets of product-related impurities include non-potent rAAV capsid (empty rAAV, partially filled rAAV, rAAV capsid containing hcDNA insert or portion of plasmid DNA), capsid-capsid complexes and capsid-DNA complexes [2]Gagnon P, Leskovec M, Goricar B, Štrancar A. Streamlining Industrial Purification of Adeno-Associated Virus. Bioprocess. Int. 2020; 18(11–12), 1–4. [5]Penaud-Budloo M, François A, Clément N, Ayuso E. Pharmacology of Recombinant Adeno-associated Virus Production. Mol. Ther. Methods Clin. Dev. 2018; 8, 166–180. [6]Wright JF. Product-related impurities in clinical-grade recombinant AAV vectors: Characterization and risk assessment. Biomedicines 2014; 2(1), 80–97.. Fully scalable monolith based rAAV downstream platforms can eliminate both process-specific and product-specific impurities. An optimized rAAV production process and sample pre-treatment further raise the quality of input material for monolith chromatography.
The following article shares experimental data showing how the processes of rAAV production (upstream) and purification (downstream) can be designed and optimized to achieve high viral titer, low impurities together with a sufficient percentage of full capsids. To enable process development and in-process control fast, reliable and cost-efficient, as well orthogonal to PCR and ELISA, analytics using high pressure liquid chromatography (HPLC) is needed [7]Gagnon P, Goricar B, Mencin N et al. Multiple-monitor HPLC assays for rapid process development, in-process monitoring, and validation of AAV production and purification. Pharmaceutics 2021; 13(1), 1–14. [8]Fu X, Chen WC, Argento C et al. Analytical Strategies for Quantification of Adeno-Associated Virus Empty Capsids to Support Process Development. Hum. Gene Ther. Methods 2019; 30(4), 144–152..
Increase the productivity of downstream with controlled AAV production
One of the key goals of rAAV upstream process development is achieving high viral titer together with a sufficient percentage of full capsids. Physical titer can nowadays be efficiently measured with digital or qPCR starting from relatively crude sample. However, methods that can reliably address empty/full rAAV ratio, such as AUC, SEC-MALS and TEM [8]Fu X, Chen WC, Argento C et al. Analytical Strategies for Quantification of Adeno-Associated Virus Empty Capsids to Support Process Development. Hum. Gene Ther. Methods 2019; 30(4), 144–152., would all require sample purification and concentration, in some cases for multiple logs, in order for sample to fall within their detection range. For that reason, the most used method for empty/full rAAV ratio assessment in crude upstream samples is a combination of ddPCR and ELISA data. These two methods differ in sample preparation requirements and basic principles for detecting the target, thus limiting the reliability of the obtained information. ddPCR/ELISA is also time consuming, and therefore less useful for rAAV in-process control in real time.
To overcome these limitations, PATfixTM Valve Switch (PATfix VS) analytical method was developed based on ion-exchange high performance liquid chromatography (IEX-HPLC) (Figure 2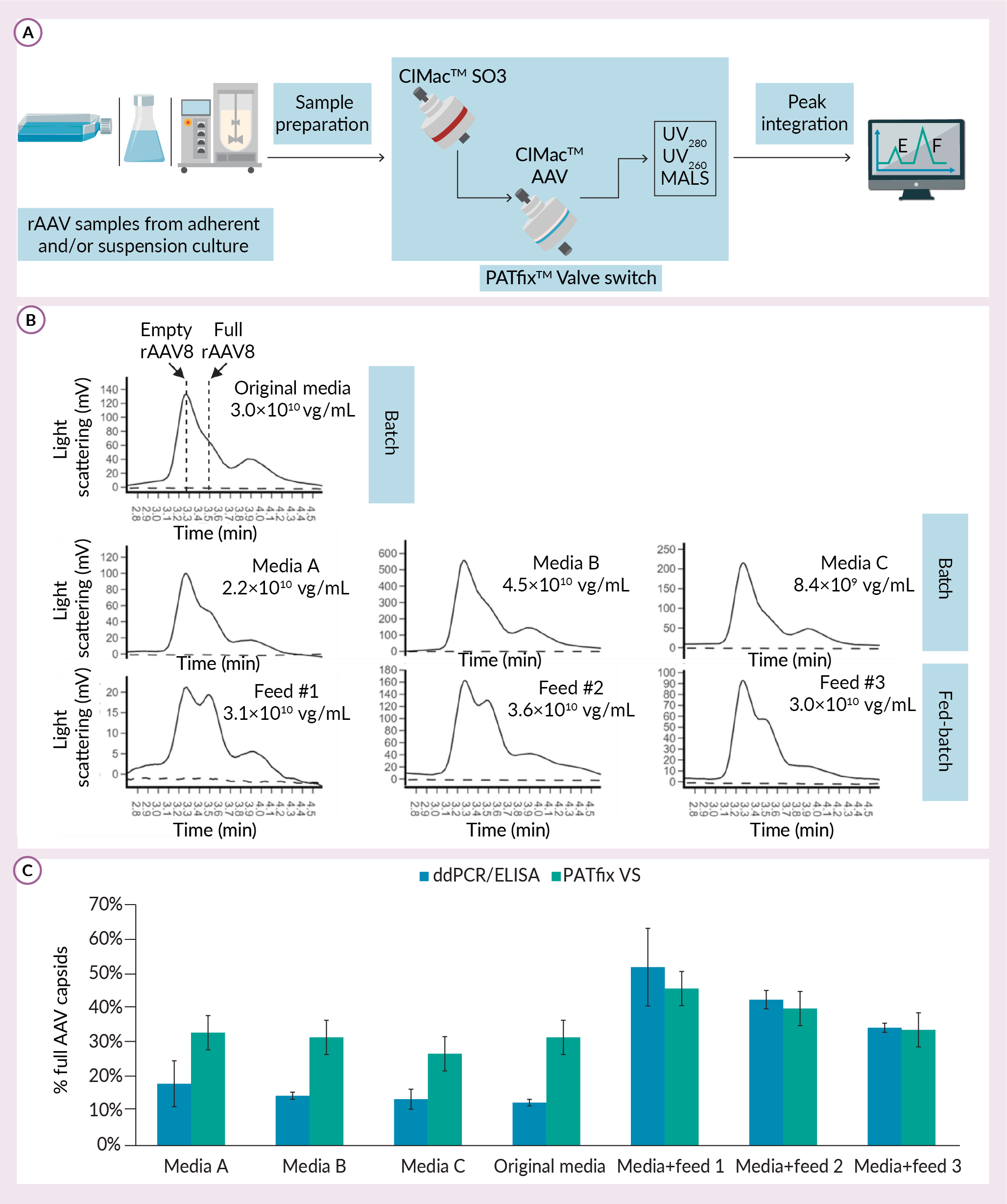 Analysis of empty and full rAAV8 capsid production in different USP media (in different dilutions).A) Scheme of PATfix Valve Switch analytical method that is used as analytical tool for batch and fed-batch screening. B) Chromatograms show zoom-in view of elution gradient on CIMac AAV column, where empty and full capsids (marked with dashed line and arrow) are visualised using MALS. Percentage of full capsids calculated from ddPCR/ELISA approach and PATfix VS are compared for listed samples. C) Standard deviation of ddPCR/ELISA is calculated as per two independent functions with Gaussian distribution. For PATfix VS standard deviation is a value of 5% which was measured experimentally for repeated analysis of AAV8 standard.A). This is an automatized two-column analytics, in which acidified and clarified samples are first captured on CIMacTM SO3 column, and then redirected to CIMac AAV column, on which empty and full rAAV capsids are separated along the salt gradient. This system is equipped with multiple detectors, including UV and Multi-Angle Light Scattering (MALS), that provide chromatograms for each sample. Selective quantification of empty and full capsid populations is done through PATfix software that calculates surface area of respective peaks. With this approach, both empty and full rAAVs can be recorded using the same detector in a single measurement of the sample.
Analysis of empty and full rAAV8 capsid production in different USP media (in different dilutions).A) Scheme of PATfix Valve Switch analytical method that is used as analytical tool for batch and fed-batch screening. B) Chromatograms show zoom-in view of elution gradient on CIMac AAV column, where empty and full capsids (marked with dashed line and arrow) are visualised using MALS. Percentage of full capsids calculated from ddPCR/ELISA approach and PATfix VS are compared for listed samples. C) Standard deviation of ddPCR/ELISA is calculated as per two independent functions with Gaussian distribution. For PATfix VS standard deviation is a value of 5% which was measured experimentally for repeated analysis of AAV8 standard.A). This is an automatized two-column analytics, in which acidified and clarified samples are first captured on CIMacTM SO3 column, and then redirected to CIMac AAV column, on which empty and full rAAV capsids are separated along the salt gradient. This system is equipped with multiple detectors, including UV and Multi-Angle Light Scattering (MALS), that provide chromatograms for each sample. Selective quantification of empty and full capsid populations is done through PATfix software that calculates surface area of respective peaks. With this approach, both empty and full rAAVs can be recorded using the same detector in a single measurement of the sample.
Due to its low limit of detection and sample volume required (less than 1×1010 of total capsids, up to 10 mL of sample), PATfix VS is suitable for early upstream process development. This is achieved with the use of improved MALS detector that functions well with relatively impure samples and has better sensitivity than UV. We used the advantage of this property for screening of different chemically defined media and feeds in rAAV8 upstream process optimization. For that experiment, cells were previously adjusted to each media, and rAAVs were produced through triple transfection in batch and fed-batch mode (Figure 2B). Compared to batch process in original media, only media B batch and original media + feed number two fed-batch resulted in titer increase. With regards to empty and full rAAV8 ratio, batch runs showed from none to modest improvement, while two fed-batch conditions incorporating feed number two and feed number one resulted in a visible increase of percentage full capsids, from 31 to 40 and 45%, respectively. Results obtained with ddPCR/ELISA method were well aligned with PATfix versus with respect to fed-batch, but not with batch samples (Figure 2C). Taken together, these results point towards partial alignment of two approaches for detecting empty/full capsid ratio. Discrepancies in PCR/ELISA were described before where poor accuracy and excessive variability were observed [8]Fu X, Chen WC, Argento C et al. Analytical Strategies for Quantification of Adeno-Associated Virus Empty Capsids to Support Process Development. Hum. Gene Ther. Methods 2019; 30(4), 144–152. [9]Wang C, Mulagapati SHR, Chen Z et al. Developing an Anion Exchange Chromatography Assay for Determining Empty and Full Capsid Contents in AAV6.2. Mol. Ther. Methods Clin. Dev. 2019; 15, 257–263.. Regardless of the method used, improvement of titer and % of full capsids were not improved with a single approach tested in this experiment (Figure 2B & 2C). Nevertheless, upstream process optimization should be directed towards maximization of both parameters [10]P. Dekleva et al. Application of PATfix Valve Switch analytics in AAV8 production media screening. .
There are other factors known to affect the empty/full rAAV ratio, such as cell line type, viable cell density, conditions of transfection, plasmid DNA, transfection reagent and others, that can be optimized using PATfix VS analytics as a guide [10]P. Dekleva et al. Application of PATfix Valve Switch analytics in AAV8 production media screening. . Moreover, the same method is applicable as at-line in-process analytics during production runs. Based on analysis of samples taken at different time points after transfection, operator can adjust the duration of the process by determining the point of time when production slows down, or the empty/full ratio starts becoming unfavorable.
Due to its robustness the method was successfully applied for analysis of empty/full ratios in complex upstream samples as well in early downstream steps, such as for example pre-capture TFF (data not shown). This allows for link-up between upstream and downstream to create more holistic approach to AAV bioprocess [10]P. Dekleva et al. Application of PATfix Valve Switch analytics in AAV8 production media screening. .
Optimizing process conditions for serotype independent capture step
Downstream starts with collection of supernatant or lysis of cell culture in bioreactor. The next main goal is to reduce host cell impurities in the process of pre-capture. TFF is usually used for the pre-capture step, but to achieve the best process yield and product purity for selected upstream and serotype, one should test a wide toolbox of pre-capture methods or, if sample is very complex, even a combination of them, including – OH chromatography, flocculation, TFF, solid phase extraction, and/or nuclease treatment [2]Gagnon P, Leskovec M, Goricar B, Štrancar A. Streamlining Industrial Purification of Adeno-Associated Virus. Bioprocess. Int. 2020; 18(11–12), 1–4. [11]Rieser R, Koch J, Faccioli G et al. Comparison of different liquid chromatography-based purification strategies for adeno-associated virus vectors. Pharmaceutics 2021; 13(5). .When performing capture step on monolithic columns, acidic precipitation of impurities is a part of AAV process.
Next step in the downstream process when using monoliths is the capture step using a cation exchanger in which rAAV is bound while most of the contaminants are removed [2]Gagnon P, Leskovec M, Goricar B, Štrancar A. Streamlining Industrial Purification of Adeno-Associated Virus. Bioprocess. Int. 2020; 18(11–12), 1–4. [11]Rieser R, Koch J, Faccioli G et al. Comparison of different liquid chromatography-based purification strategies for adeno-associated virus vectors. Pharmaceutics 2021; 13(5). [12]Zabaleta N, Dai W, Bhatt U et al. Immunogenicity of an AAV-based, room-temperature stable, single dose COVID-19 vaccine in mouse and non-human primates. bioRxiv [Preprint].Update in: Cell Host Microbe 2021; 29(9), 1437–1453.e8.. On a CIMmultusTM SO3 column, majority of proteins are removed in CIP peak while small portion also in flowthrough during the sample application step. This results in better performance of the polishing step. rAAV binding is performed at pH 3.5 and elution is achieved with ascending salt concentration (Figure 3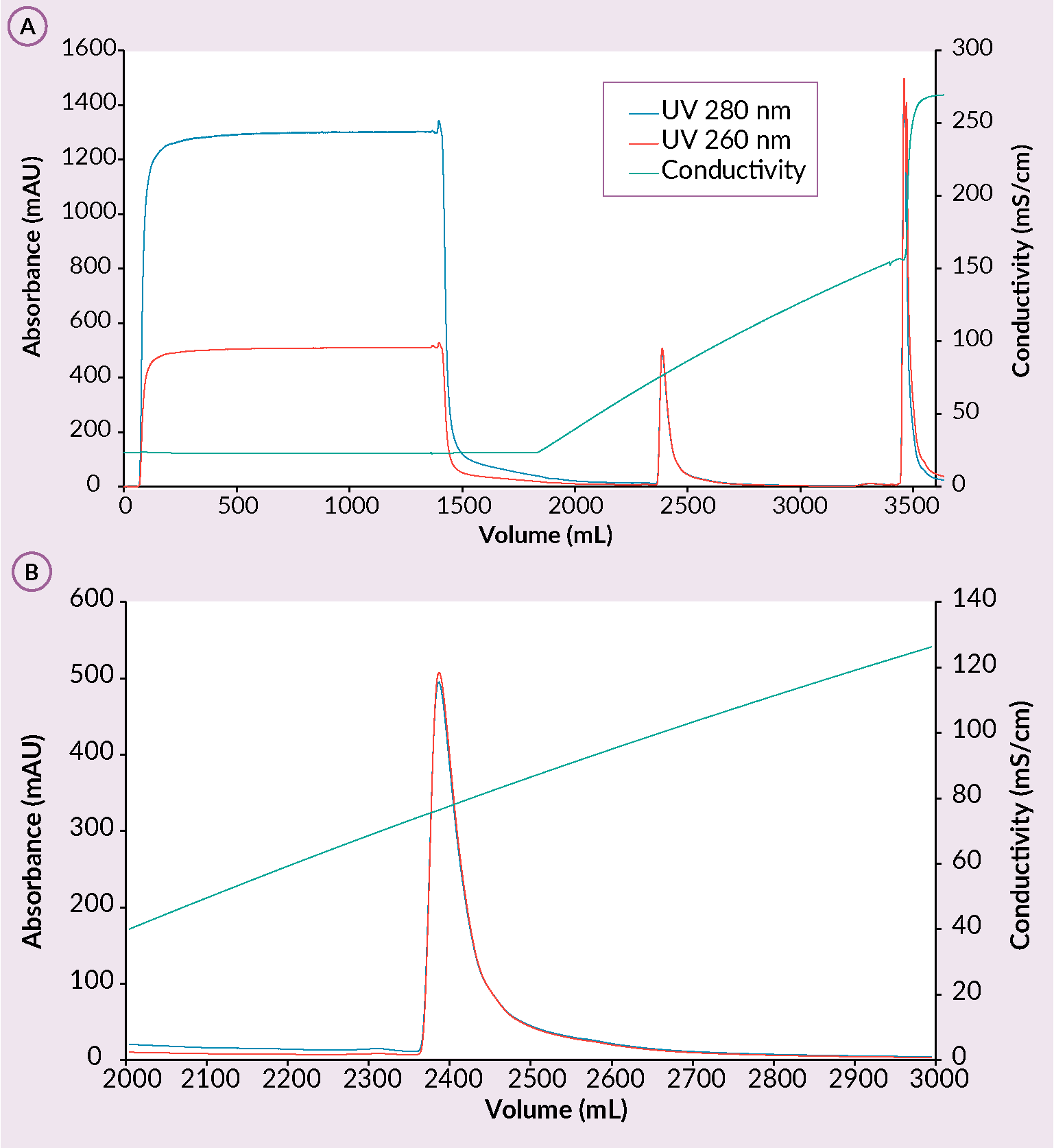 (A) Example of a CIMmultus SO3 chromatogram and (B) a zoom in on the elution.Conditions: sample AAV2/8 mobile phase A: 50 mM formic acid, 0.2 M NaCl, 1% sucrose, 0.1% poloxamer 188, pH 3.5. mobile phase B: 50 mM formic acid, 2 M NaCl, 1% sucrose, 0.1% poloxamer 188, pH 3.5, column CIMmultus SO3-80mL, method A to 100%B in 20CV, 3CV CIP (1M NaOH, 2M NaCl).). Due to the structural nature of the monolith, rAAV does not succumb to shear stress which allows the usage of high flowrates. As a result of a high binding capacity of the column, rAAV is concentrated to target levels (1×1013 to 5×1013 vg/ml). Last but not least, the cation exchange monolithic columns allow for capturing of any serotype or AAV chimera, making these columns a perfect tool for AAV library screening [2]Gagnon P, Leskovec M, Goricar B, Štrancar A. Streamlining Industrial Purification of Adeno-Associated Virus. Bioprocess. Int. 2020; 18(11–12), 1–4. [11]Rieser R, Koch J, Faccioli G et al. Comparison of different liquid chromatography-based purification strategies for adeno-associated virus vectors. Pharmaceutics 2021; 13(5). [12]Zabaleta N, Dai W, Bhatt U et al. Immunogenicity of an AAV-based, room-temperature stable, single dose COVID-19 vaccine in mouse and non-human primates. bioRxiv [Preprint].Update in: Cell Host Microbe 2021; 29(9), 1437–1453.e8. [13]Ye GJ, Budzynski E, Sonnentag P et al. Safety and Biodistribution Evaluation in Cynomolgus Macaques of rAAV2tYF-CB-hRS1, a Recombinant Adeno-Associated Virus Vector Expressing Retinoschisin. Hum. Gene Ther. Clin. Dev. 2015; 26(3), 165–176. [14]Bažec K, Krašna M, Leskovec M, Tajnik Sbaizero M. CIM SO3 Monolithic 96-well Plate for fast and efficient rAAV capture step screening after Kryptonase-TFF pretreatment; Available at: Accessed: Oct. 12 2022.
(A) Example of a CIMmultus SO3 chromatogram and (B) a zoom in on the elution.Conditions: sample AAV2/8 mobile phase A: 50 mM formic acid, 0.2 M NaCl, 1% sucrose, 0.1% poloxamer 188, pH 3.5. mobile phase B: 50 mM formic acid, 2 M NaCl, 1% sucrose, 0.1% poloxamer 188, pH 3.5, column CIMmultus SO3-80mL, method A to 100%B in 20CV, 3CV CIP (1M NaOH, 2M NaCl).). Due to the structural nature of the monolith, rAAV does not succumb to shear stress which allows the usage of high flowrates. As a result of a high binding capacity of the column, rAAV is concentrated to target levels (1×1013 to 5×1013 vg/ml). Last but not least, the cation exchange monolithic columns allow for capturing of any serotype or AAV chimera, making these columns a perfect tool for AAV library screening [2]Gagnon P, Leskovec M, Goricar B, Štrancar A. Streamlining Industrial Purification of Adeno-Associated Virus. Bioprocess. Int. 2020; 18(11–12), 1–4. [11]Rieser R, Koch J, Faccioli G et al. Comparison of different liquid chromatography-based purification strategies for adeno-associated virus vectors. Pharmaceutics 2021; 13(5). [12]Zabaleta N, Dai W, Bhatt U et al. Immunogenicity of an AAV-based, room-temperature stable, single dose COVID-19 vaccine in mouse and non-human primates. bioRxiv [Preprint].Update in: Cell Host Microbe 2021; 29(9), 1437–1453.e8. [13]Ye GJ, Budzynski E, Sonnentag P et al. Safety and Biodistribution Evaluation in Cynomolgus Macaques of rAAV2tYF-CB-hRS1, a Recombinant Adeno-Associated Virus Vector Expressing Retinoschisin. Hum. Gene Ther. Clin. Dev. 2015; 26(3), 165–176. [14]Bažec K, Krašna M, Leskovec M, Tajnik Sbaizero M. CIM SO3 Monolithic 96-well Plate for fast and efficient rAAV capture step screening after Kryptonase-TFF pretreatment; Available at: Accessed: Oct. 12 2022.
The process of finding the best possible condition for SO3 chromatography can be time consuming since different parameters must be optimized to ensure the best possible binding of rAAV to the matrix. To speed up the process of screening many samples or conditions, a standard 96-well design offers a great advantage. CIM® SO3 0.05 mL Monolithic 96-well plates have been developed for this purpose.
An experiment was performed to test different pre-treatment methods and screen buffers of different pH, sodium chloride concentrations and use of Poloxamer 188 to determine the optimal combination of parameters for the SO3 capture. Three different pre-treatment options were explored – acidification, TFF and TFF coupled with salt tolerant DNase. For the used sample the TFF coupled with salt tolerant DNase was identified as the best pre-purification step and was used to screen buffers using CIM SO3 0.05 mL Monolithic 96-well plates. DNase-treated TFF retentate rAAV2/9 was acidified, filtered, and loaded to the CIM SO3 0.05 mL monolithic 96-well plate. Plate was washed with different mobile phases A (MPA; different pH, sodium chloride and poloxamer conc.). Elution was performed using 4 CV of mobile phase B (MPB; buffers with corresponding pH and poloxamer conc. with 2 M NaCl). To determine optimal combination of parameters for the SO3 capture step wash and elution fractions were analysed using fluorescence microplate reader, ELISA and SDS-PAGE [14]Bažec K, Krašna M, Leskovec M, Tajnik Sbaizero M. CIM SO3 Monolithic 96-well Plate for fast and efficient rAAV capture step screening after Kryptonase-TFF pretreatment; Available at: Accessed: Oct. 12 2022.
Fluorescence readings (FLD) of elution and wash samples from the buffer screen experiment on the SO3 plates showed stronger signal in wells where the rAAV was detected. For wash fractions, wells with higher pH and/or sodium chloride concentration in mobile phase A showed inefficient binding observed as high fluorescence signal. On the other hand, higher FLD values in elution fractions correlate with successful rAAV binding. Efficiency of rAAV capture was verified with capsid specific ELISA analytics (Figure 4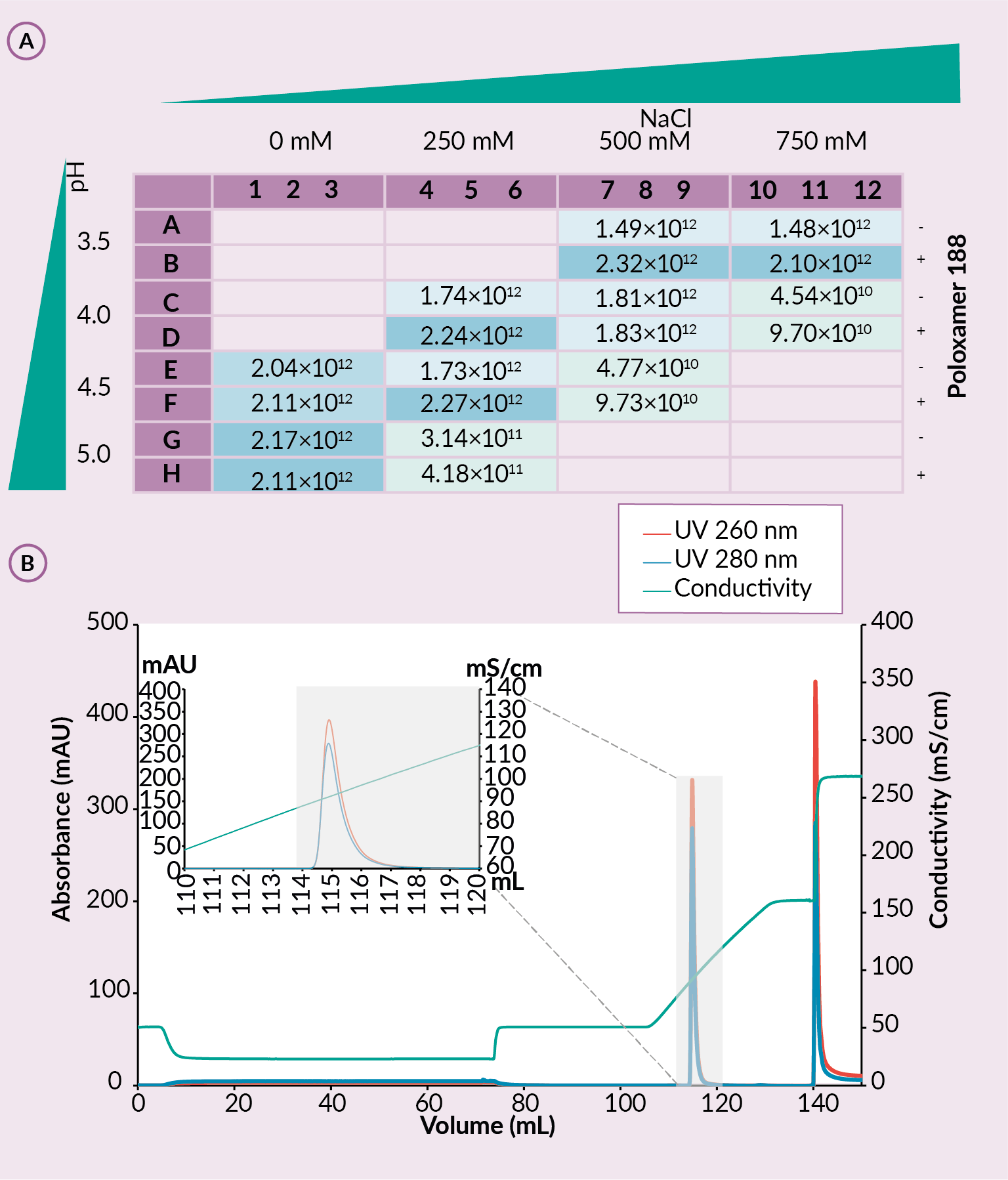 (A) ELISA analytics of elution fractions for rAAV titer and (B) elution from CIMmultus using optimized conditions.Conditions: sample AAV2/9. mobile phase A: 20 mM formic acid, 0.5 M NaCl with the presence of poloxamer 188, pH 3.5. mobile phase B: 20 mM formic acid, 2 M NaCl, with the presence of poloxamer 188, pH 3.5, column CIMmultus SO3-1mL, method A to 100%B in 25CV, then step to 100% B in 10CV, 3CV CIP (1M NaOH, 2M NaCl).A) and the impurity profile with SDS-PAGE [14]Bažec K, Krašna M, Leskovec M, Tajnik Sbaizero M. CIM SO3 Monolithic 96-well Plate for fast and efficient rAAV capture step screening after Kryptonase-TFF pretreatment; Available at: Accessed: Oct. 12 2022.
(A) ELISA analytics of elution fractions for rAAV titer and (B) elution from CIMmultus using optimized conditions.Conditions: sample AAV2/9. mobile phase A: 20 mM formic acid, 0.5 M NaCl with the presence of poloxamer 188, pH 3.5. mobile phase B: 20 mM formic acid, 2 M NaCl, with the presence of poloxamer 188, pH 3.5, column CIMmultus SO3-1mL, method A to 100%B in 25CV, then step to 100% B in 10CV, 3CV CIP (1M NaOH, 2M NaCl).A) and the impurity profile with SDS-PAGE [14]Bažec K, Krašna M, Leskovec M, Tajnik Sbaizero M. CIM SO3 Monolithic 96-well Plate for fast and efficient rAAV capture step screening after Kryptonase-TFF pretreatment; Available at: Accessed: Oct. 12 2022.
The most optimal combination of parameters for mobile phase A from the CIM SO3 0.05 mL Monolithic 96-well plates was chosen – pH 3.5 and 500 mM NaCl in presence of Poloxamer 188. This mobile phase A was further used for experiments on CIMmultus (Figure 4B). It was shown that sample obtained by TFF with salt tolerant DNase coupled with the most optimal mobile phase A gave the highest vector recovery, highest protein/DNA reduction and highest dynamic binding capacity. SO3 step recovery was 87.70%, while protein and DNA content were reduced by 99,98% and 99,25%, respectively. Dynamic binding capacity was approximately 1.44×1014 capsids per milliliter of SO3 column. This confirmed successful optimization of the capture step [14]Bažec K, Krašna M, Leskovec M, Tajnik Sbaizero M. CIM SO3 Monolithic 96-well Plate for fast and efficient rAAV capture step screening after Kryptonase-TFF pretreatment; Available at: Accessed: Oct. 12 2022.
CIM SO3 0.05 mL Monolithic 96-well plates are efficient and fast tool for rAAV capture step screening and fine tuning of mobile phases, including automating of procedure and analytics steps. Obtained results can be applied to CIMmultus preparative line, which is scalable to large industrial volumes.
Different chromatographic possibilities for full enrichment. Separating empty and partial capsids from full AAV
In each AAV downstream process, one of the key steps is enrichment of full capsids. Usually, only a part of rAAV capsids are functional capsids. Besides ultracentrifugation the most common approach is liquid chromatography using ion exchange chemistries, which is based on particles’ charge differences. It enables separation of the full (F) capsids and product related impurities including non-functioning AAV capsids (empty, partially filled, misfolded and wrongly packaged genome or other DNA containing subspecies) [15]Dickerson R, Argento C, Pieracci J, Bakhshayeshi M. Separating Empty and Full Recombinant Adeno‐Associated Virus Particles Using Isocratic Anion Exchange Chromatography. Biotechnol. J. 2021; 16(1), 2000015. [16]Joshi PRH, Bernier A, Moço PD, Schrag J, Chahal PS, Kamen A. Development of a scalable and robust AEX method for enriched rAAV preparations in genome-containing VCs of serotypes 5, 6, 8, and 9. Mol. Ther. Methods Clin. Dev. 2021; 21, 341–356. [17]Bingnan Gu, Vijetha Bhat, Wen Dong et al. Establishment of a scalable manufacturing platform for in-silico-derived ancestral adeno-associated virus vectors, Cell Gene Ther. Insights 2018; 4(S1), pp. 753–769..
Quaternary amine (QA) is well known and several times patented method [18]Lock M, Alvira M. Scalable purification method for AAV9 (WO2017160360A9) 2017. [19]Lock M, Vandenberghe LH, Wilson JM. Scalable production method for AAV (US20160040137A1) 2016. for the separation of empty and full capsids. CIMmultus™ QA monolithic columns exploit anion exchange mechanism; sample is loaded onto the column at low conductivity (2–5 mS/cm) and elution is usually achieved with ascending salt concentration. pH ranging from 8.5 – 9.5 should be used (Figure 5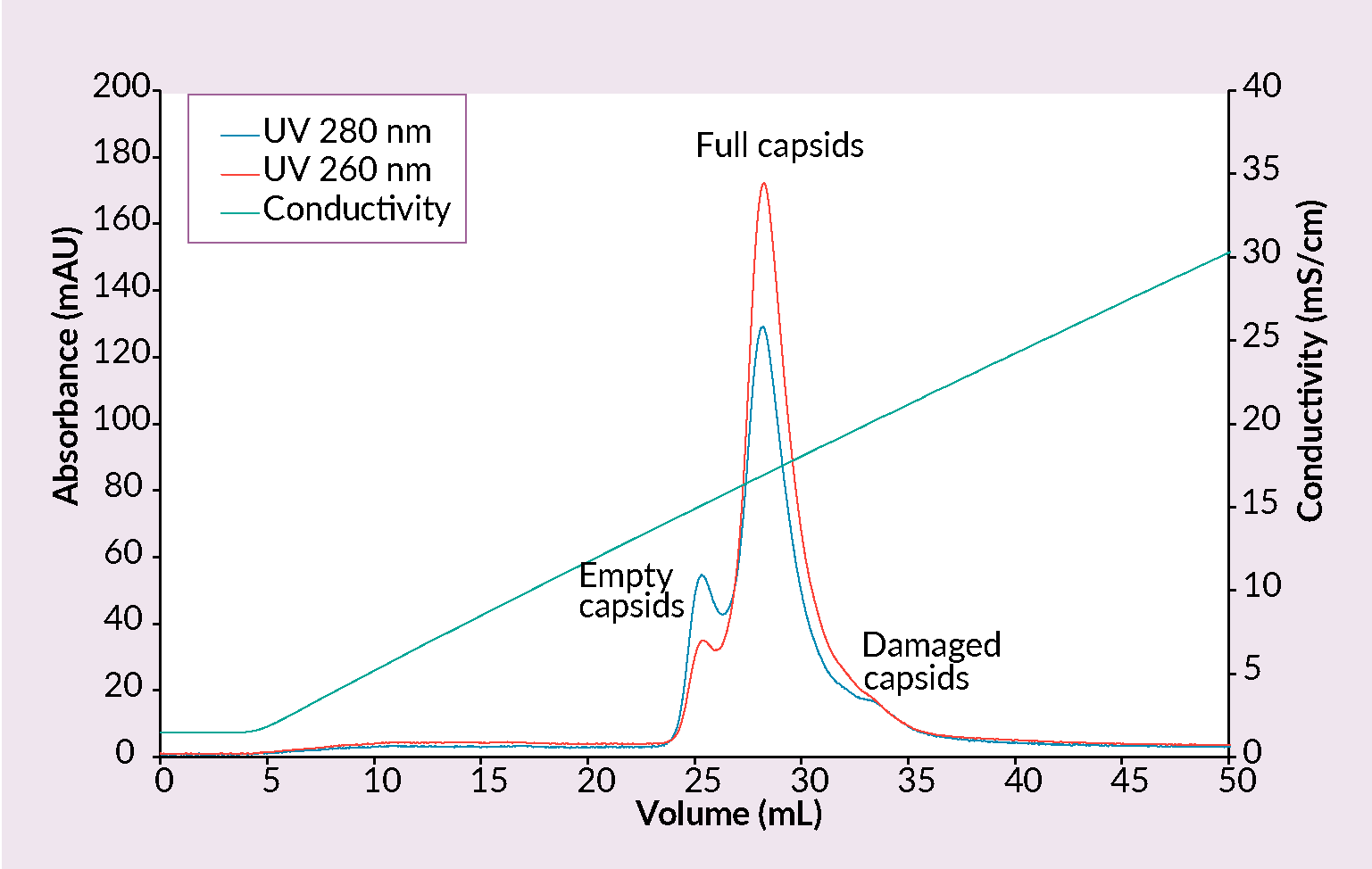 Separation of empty and full AAV capsids by anion exchange chromatography with a salt gradient.Conditions: sample AAV2/8 (SO3 eluate), mobile phase A: 25 mM BTP, 1% sucrose, 0.1% poloxamer 188, pH 9.0, mobile phase B: 25 mM BTP, 0.5 M KCl, 1% sucrose, 0.1% poloxamer 188, pH 9.0, column CIMmultus QA- 1mL, method A to 50% B in 50CV, 5CV CIP (1M NaOH, 2M NaCl).). Presence of magnesium has effect on elution and removal of empty capsids.
Separation of empty and full AAV capsids by anion exchange chromatography with a salt gradient.Conditions: sample AAV2/8 (SO3 eluate), mobile phase A: 25 mM BTP, 1% sucrose, 0.1% poloxamer 188, pH 9.0, mobile phase B: 25 mM BTP, 0.5 M KCl, 1% sucrose, 0.1% poloxamer 188, pH 9.0, column CIMmultus QA- 1mL, method A to 50% B in 50CV, 5CV CIP (1M NaOH, 2M NaCl).). Presence of magnesium has effect on elution and removal of empty capsids.
New ligands were explored to provide researchers with alternative options if the QA does not provide satisfactory full rAAV enrichment. Therefore, to create new opportunities for eliminating empty and other non-functioning AAV capsids from rAAV preparations CIMmultus PrimaS™ (Figure 6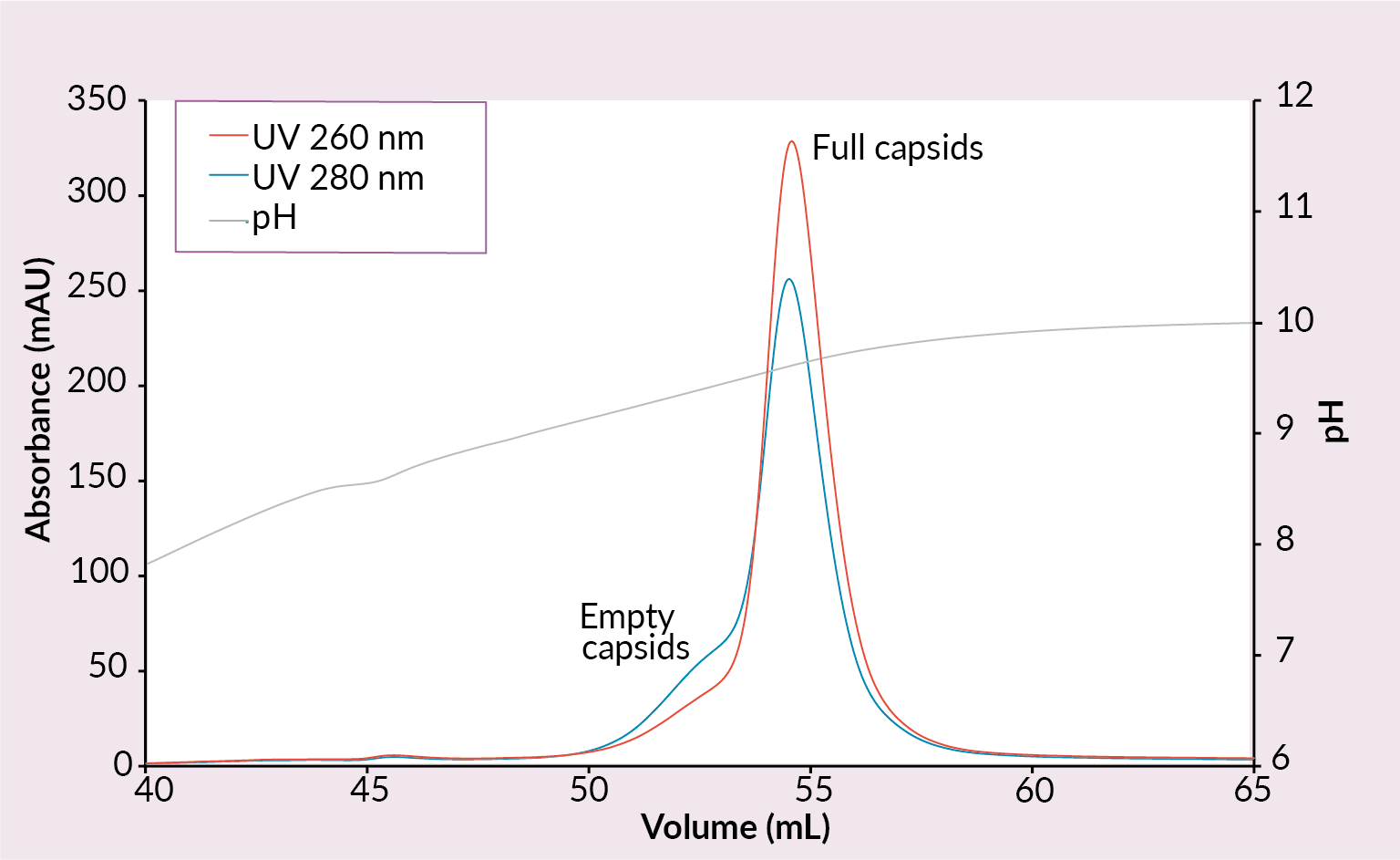 Example of a CIMmultus PrimaS chromatogram.Conditions: sample AAV2/8 (SO3 eluate), mobile phase A: 10 mM Tris, 10 mM BTP, 2 mM MgCl2, 1% saccharose, 0.1% poloxamer pH 7.00, mobile phase B: 10 mM Tris, 10 mM BTP, 2 mM MgCl2, 1% saccharose, 0.1% poloxamer pH 10, column CIMmultus PrimaS-1mL, method A to 100%B in 30CV, 5CV CIP (0.1M NaOH).) and CIMmultus™ PrimaT columns were developed (Figure 7
Example of a CIMmultus PrimaS chromatogram.Conditions: sample AAV2/8 (SO3 eluate), mobile phase A: 10 mM Tris, 10 mM BTP, 2 mM MgCl2, 1% saccharose, 0.1% poloxamer pH 7.00, mobile phase B: 10 mM Tris, 10 mM BTP, 2 mM MgCl2, 1% saccharose, 0.1% poloxamer pH 10, column CIMmultus PrimaS-1mL, method A to 100%B in 30CV, 5CV CIP (0.1M NaOH).) and CIMmultus™ PrimaT columns were developed (Figure 7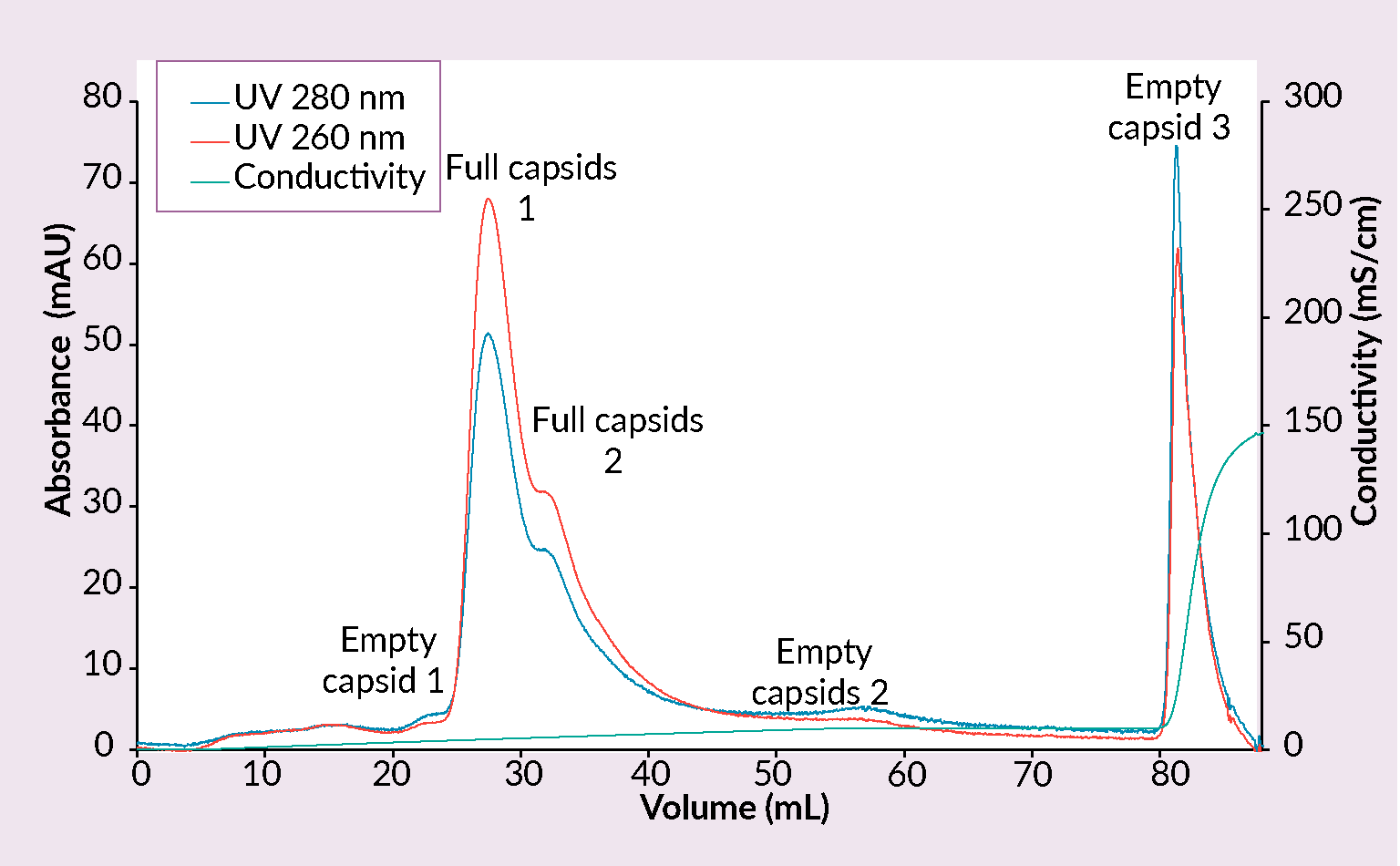 Example of a CIMmultus PrimaT chromatogram.Conditions: sample AAV2/8 (SO3 eluate), mobile phase A1: 25 mM HEPES, 1% sucrose, 0.1% poloxamer pH 7.0, mobile phase A2: 50 mM Tris, 13.6 mM borate, 1% sucrose, 0.1% poloxamer pH 9.0, mobile phase B1: 50 mM Tris, 9.6 mM borate, 50 mM MgCl2, 1% sucrose, 0.1% poloxamer pH 9.0, mobile phase B2: 50 mM Tris, 12 mM borate, 2 M NaCl, 1% sucrose, 0.1% poloxamer pH 9.0, column CIMmultus PrimaT 1mL, method A2 to 100% B1 in 50CV, then step to 100% B2 for 10CVs, 5CV CIP (1M NaOH, 2M NaCl).).
Example of a CIMmultus PrimaT chromatogram.Conditions: sample AAV2/8 (SO3 eluate), mobile phase A1: 25 mM HEPES, 1% sucrose, 0.1% poloxamer pH 7.0, mobile phase A2: 50 mM Tris, 13.6 mM borate, 1% sucrose, 0.1% poloxamer pH 9.0, mobile phase B1: 50 mM Tris, 9.6 mM borate, 50 mM MgCl2, 1% sucrose, 0.1% poloxamer pH 9.0, mobile phase B2: 50 mM Tris, 12 mM borate, 2 M NaCl, 1% sucrose, 0.1% poloxamer pH 9.0, column CIMmultus PrimaT 1mL, method A2 to 100% B1 in 50CV, then step to 100% B2 for 10CVs, 5CV CIP (1M NaOH, 2M NaCl).).
CIMmultus PrimaS is a multimodal ligand which exploits a unique combination of weak anion exchange and hydrogen bonding that achieves different selectivity compared to the QA ligand [11]Rieser R, Koch J, Faccioli G et al. Comparison of different liquid chromatography-based purification strategies for adeno-associated virus vectors. Pharmaceutics 2021; 13(5). [20]P. Gagnon, B. Goričar, S. D. Prebil, H. Jug, M. Leskovec, and A. Štrancar. Separation of Empty and Full Adeno-Associated Virus Capsids from a Weak Anion Exchanger by Elution with an Ascending pH Gradient at Low Ionic Strength. 2021; Available at; Accessed: May 25, 2022. Binding is performed at low conductivity (2.5 mS/cm) with the presence of magnesium (2mM), while the best separation is achieved when eluted with ascending pH gradient (pH from 7 – 10) at low conductivity.
CIMmultus PrimaT is a second novel multimodal column which enables AAV sub-species separation. Sample heterogeneity poses a cumbersome challenge on downstream process to isolate and purify only the active drug substance. Accounting only on vector genome estimation might mislead and result in pooling not only potent intact full AAV capsids, but also product related impurities. Various factors such as AAV serotype; expression system which produces a combination of AAV subpopulation species; as well as glycosylation and phosphorylation of capsids, all contribute to slight charge variations. Relying only on charge differences, using ion exchange columns, results in diminished or absence of resolution between the capsids subspecies. To overcome limitations posed by charge separation, CIMmultus PrimaT was developed.
CIMmultus PrimaT funtions as a weak-anion exchanger with hydrogen bond properties and metal affinity coordination effect. Two different approaches are recommended for the elution of AAV capsids: first, a linear MgCl2 gradient where the full AAV particles elute and then a high salt wash step where most of the empty particles elute [21]Gagnon P, Leskovec M, Prebil SD et al. Removal of empty capsids from adeno-associated virus preparations by multimodal metal affinity chromatography. J. Chromatogr. A. 2021; 1649, 462210. .
The PrimaS and PrimaT ligands are available on analytical scale as well.
Analytics of Prima T polishing
To test the ability of CIMmultus PrimaT to distinguish AAV subspecies, AAV 2/8 serotype sample captured by cation exchange chromatography (CIMmultus SO3), was buffer exchanged and loaded on CIMmultus PrimaT. SO3 eluate consisted of 47% full AAV capsids before the enrichment step. A novel column CIMmultus PrimaT provided significant evidence for ability of AAV subspecies separation (Figure 8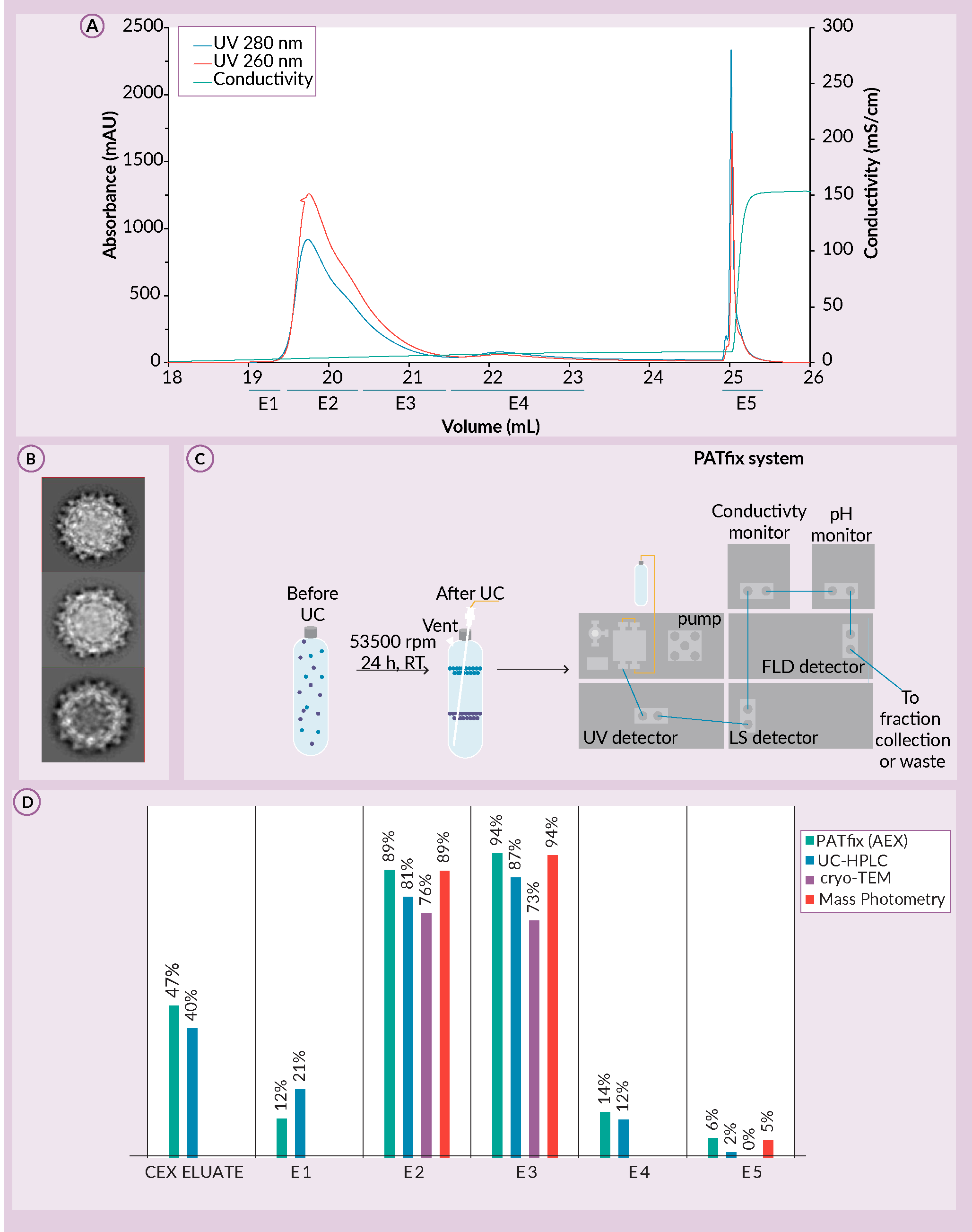 A) Preparative chromatogram for CIMmultus PrimaT. E1 to E5 represent fractions taken and assessed by orthogonal analytics. B) Cryo-TEM micrographs for fractions E2, E3 and E5. C) The UC-HPLC system. D) Percentage of full AAV capsids based on PATfix HPLC AEX-FP-TRP, UC-HPLC, Cryo-TEM and mass photometry assays.A). Characterization of individual PrimaT fractions by orthogonal analytics revealed that there are at least three subpopulations of empty and two subpopulations of full AAV capsids, similar in both charge and density [22]R. Žigon et al. Introduction Characterization of various AAV subpopulation species, separated by multimodal column PrimaT’; Available at; .
A) Preparative chromatogram for CIMmultus PrimaT. E1 to E5 represent fractions taken and assessed by orthogonal analytics. B) Cryo-TEM micrographs for fractions E2, E3 and E5. C) The UC-HPLC system. D) Percentage of full AAV capsids based on PATfix HPLC AEX-FP-TRP, UC-HPLC, Cryo-TEM and mass photometry assays.A). Characterization of individual PrimaT fractions by orthogonal analytics revealed that there are at least three subpopulations of empty and two subpopulations of full AAV capsids, similar in both charge and density [22]R. Žigon et al. Introduction Characterization of various AAV subpopulation species, separated by multimodal column PrimaT’; Available at; .
Additional analytics were performed to estimate the percentage of full AAV capsids in elution samples. One of them was UC-HPLC. During ultracentrifugation, capsid populations separate based on their density, with full capsids at the bottom and empty capsids at the top of the CsCl gradient [23]Peljhan S, Štokelj M, Prebil SD, Gagnon P, Štrancar A. Multiple-parameter profiling of density gradient ultracentrifugation for characterization of empty and full capsid distribution in AAV preparations. Cell Gene Ther. Insights 2021; 7(2), 161–169.. Fractions can be collected after ultracentrifugation for analysis of UV260 and UV280 signals, tryptophan intrinsic fluorescence (FL) and Multi-Angle Light Scattering (MALS) (Figure 8C). HPLC analytics estimation using PATfix correlated well with mass photometry, cryo-TEM and UC results with average values of 83% ± 7%, 87% ± 10%, and 3% ± 78% in fractions E2, E3 and E5, respectively (Figure 8D). In case of cryo-TEM, only distinctive full capsids are shown not accounting uncertain species which represent additional 11.63% (E2) and 15.93% (E3) [22]R. Žigon et al. Introduction Characterization of various AAV subpopulation species, separated by multimodal column PrimaT’; Available at; .
Our results show that novel monolithic column CIMmultus PrimaT is able to separate full AAV subspecies. In addition, novel ligands to improve full and partial AAV capsids separation are under development.
Translation Insight
Although there have been many improvements in rAAV manufacture for gene therapy with licenced therapeutics already reaching the market, there are still some challenges manufacturers face to improve the final product purity. In the process of rAAV production, high viral titers must be achieved but attention should also be paid to impurities levels. Therefore, it is imporant to implement fast and robust analytical tools for process control to analyse such complex harvest samples. Optimizing chromatographic capture step to screen for optimal conditions is time-consuming and it can be refined by using high-throughput plates. Partially purified rAAV capsids are not only empty and full, but there are also multiple species of capsids. Since the evolution of new surface chemistries and methods for polishing step is still at its beginning, emphasis must be placed on optimization to achieve the adequate separation of the active product.
References
1. Penaud-Budloo M, François A, Clément N, Ayuso E. Pharmacology of Recombinant Adeno-associated Virus Production. Mol. Ther. Methods Clin. Dev. 2018; 8, 166–180. Crossref
2. Gagnon P, Leskovec M, Goricar B, Štrancar A. Streamlining Industrial Purification of Adeno-Associated Virus. Bioprocess. Int. 2020; 18(11–12), 1–4. Crossref
3. Qu W, Wang M, Wu Y, Xu R. Scalable Downstream Strategies for Purification of Recombinant Adeno- Associated Virus Vectors in Light of the Properties. Curr. Pharm. Biotechnol. 2015; 16(8), 684–695. Crossref
4. Potter M Lins B, Mietzsch M et al. A simplified purification protocol for recombinant adeno-associated virus vectors. Mol. Ther. Methods Clin. Dev. 2014; 1, 14034. Crossref
5. Penaud-Budloo M, François A, Clément N, Ayuso E. Pharmacology of Recombinant Adeno-associated Virus Production. Mol. Ther. Methods Clin. Dev. 2018; 8, 166–180. Crossref
6. Wright JF. Product-related impurities in clinical-grade recombinant AAV vectors: Characterization and risk assessment. Biomedicines 2014; 2(1), 80–97. Crossref
7. Gagnon P, Goricar B, Mencin N et al. Multiple-monitor HPLC assays for rapid process development, in-process monitoring, and validation of AAV production and purification. Pharmaceutics 2021; 13(1), 1–14. Crossref
8. Fu X, Chen WC, Argento C et al. Analytical Strategies for Quantification of Adeno-Associated Virus Empty Capsids to Support Process Development. Hum. Gene Ther. Methods 2019; 30(4), 144–152. Crossref
9. Wang C, Mulagapati SHR, Chen Z et al. Developing an Anion Exchange Chromatography Assay for Determining Empty and Full Capsid Contents in AAV6.2. Mol. Ther. Methods Clin. Dev. 2019; 15, 257–263. Crossref
10. P. Dekleva et al. Application of PATfix Valve Switch analytics in AAV8 production media screening. Crossref
11. Rieser R, Koch J, Faccioli G et al. Comparison of different liquid chromatography-based purification strategies for adeno-associated virus vectors. Pharmaceutics 2021; 13(5). Crossref
12. Zabaleta N, Dai W, Bhatt U et al. Immunogenicity of an AAV-based, room-temperature stable, single dose COVID-19 vaccine in mouse and non-human primates. bioRxiv [Preprint].Update in: Cell Host Microbe 2021; 29(9), 1437–1453.e8. Crossref
13. Ye GJ, Budzynski E, Sonnentag P et al. Safety and Biodistribution Evaluation in Cynomolgus Macaques of rAAV2tYF-CB-hRS1, a Recombinant Adeno-Associated Virus Vector Expressing Retinoschisin. Hum. Gene Ther. Clin. Dev. 2015; 26(3), 165–176. Crossref
14. Bažec K, Krašna M, Leskovec M, Tajnik Sbaizero M. CIM SO3 Monolithic 96-well Plate for fast and efficient rAAV capture step screening after Kryptonase-TFF pretreatment; Available at: Accessed: Oct. 12 2022 Crossref
15. Dickerson R, Argento C, Pieracci J, Bakhshayeshi M. Separating Empty and Full Recombinant Adeno‐Associated Virus Particles Using Isocratic Anion Exchange Chromatography. Biotechnol. J. 2021; 16(1), 2000015. Crossref
16. Joshi PRH, Bernier A, Moço PD, Schrag J, Chahal PS, Kamen A. Development of a scalable and robust AEX method for enriched rAAV preparations in genome-containing VCs of serotypes 5, 6, 8, and 9. Mol. Ther. Methods Clin. Dev. 2021; 21, 341–356. Crossref
17. Bingnan Gu, Vijetha Bhat, Wen Dong et al. Establishment of a scalable manufacturing platform for in-silico-derived ancestral adeno-associated virus vectors, Cell Gene Ther. Insights 2018; 4(S1), pp. 753–769. Crossref
18. Lock M, Alvira M. Scalable purification method for AAV9 (WO2017160360A9) 2017. Crossref
19. Lock M, Vandenberghe LH, Wilson JM. Scalable production method for AAV (US20160040137A1) 2016. Crossref
20. P. Gagnon, B. Goričar, S. D. Prebil, H. Jug, M. Leskovec, and A. Štrancar. Separation of Empty and Full Adeno-Associated Virus Capsids from a Weak Anion Exchanger by Elution with an Ascending pH Gradient at Low Ionic Strength. 2021; Available at; Accessed: May 25, 2022 Crossref
21. Gagnon P, Leskovec M, Prebil SD et al. Removal of empty capsids from adeno-associated virus preparations by multimodal metal affinity chromatography. J. Chromatogr. A. 2021; 1649, 462210. Crossref
22. R. Žigon et al. Introduction Characterization of various AAV subpopulation species, separated by multimodal column PrimaT’; Available at; Crossref
23. Peljhan S, Štokelj M, Prebil SD, Gagnon P, Štrancar A. Multiple-parameter profiling of density gradient ultracentrifugation for characterization of empty and full capsid distribution in AAV preparations. Cell Gene Ther. Insights 2021; 7(2), 161–169. Crossref
Affiliations
Rok Žigon
Head of Product-Application Area for AAV and Adenoviruses,
Sartorius BIA Separations
Mojca Tajnik Sbaizero
Deputy Head of Process Development Department for Viruses,
Sartorius BIA Separations
Ivana Petrović Koshmak
Head of Product-Application Area for Upstream Process Development,
Sartorius BIA Separations
Veronika Fujs
Associate Scientist in the Process Development Department for
Viruses,
Sartorius BIA Separations
Maja Leskovec
Head of Process Development
Department for Viruses,
Sartorius BIA Separations
Aleš Štrancar
Managing director,
Sartorius BIA Separations
Authorship & Conflict of Interest
Contributions: All named authors take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Acknowledgements: We thank all colleagues who were involved in the research for this paper. They include Janja Merkelj Koren, Kaja Bažec, Valentina Novak, Petra Dekleva, Mirjam Krašna, Blaž Šimenc, Blaž Goričar, Ažbe Žnidaršič and Andrej Mihevc.
Many thanks to Laboratory Scale Product Department at Sartorius BIA Separations for the development of CIM Monolithic 96-well plates, Research and Development Department for the development of novel CIMmultus PrimaS and CIMmultus PrimaT columns, and Refeyn Ltd for performing mass photometry analytics.
Disclosure and potential conflicts of interest: All authors are long term employees of Sartorius BIA Separations. Portions of the work described in this publication will be presented on upcoming meetings and conferences. Participation costs will be covered by Sartorius BIA Separations. CIM is technology protected by patent. Following products might be subject to patent: CIM PrimaT, PATfix and CIM monolithinc 96-well plate.
Funding declaration: This manuscript is a result of general R&D work within Sartorius BIA Separations.
Article & copyright information
Copyright: Published by Cell and Gene Therapy Insights under Creative Commons License Deed CC BY NC ND 4.0 which allows anyone to copy, distribute, and transmit the article provided it is properly attributed in the manner specified below. No commercial use without permission.
Attribution: Copyright © 2022 Sartorius BIA Separations. Published by Cell and Gene Therapy Insights under Creative Commons License Deed CC BY NC ND 4.0.
Article source: Invited; externally peer reviewed.
Submitted for peer review: Oct 24 2022; Revised manuscript received: Oct 27 2022; Publication date: Nov 18 2022.

