Process & analytical insights for GMP manufacturing of mRNA lipid nanoparticles
Cell & Gene Therapy Insights 2022; 8(4), 621–635
DOI: 10.18609/cgti.2022.246
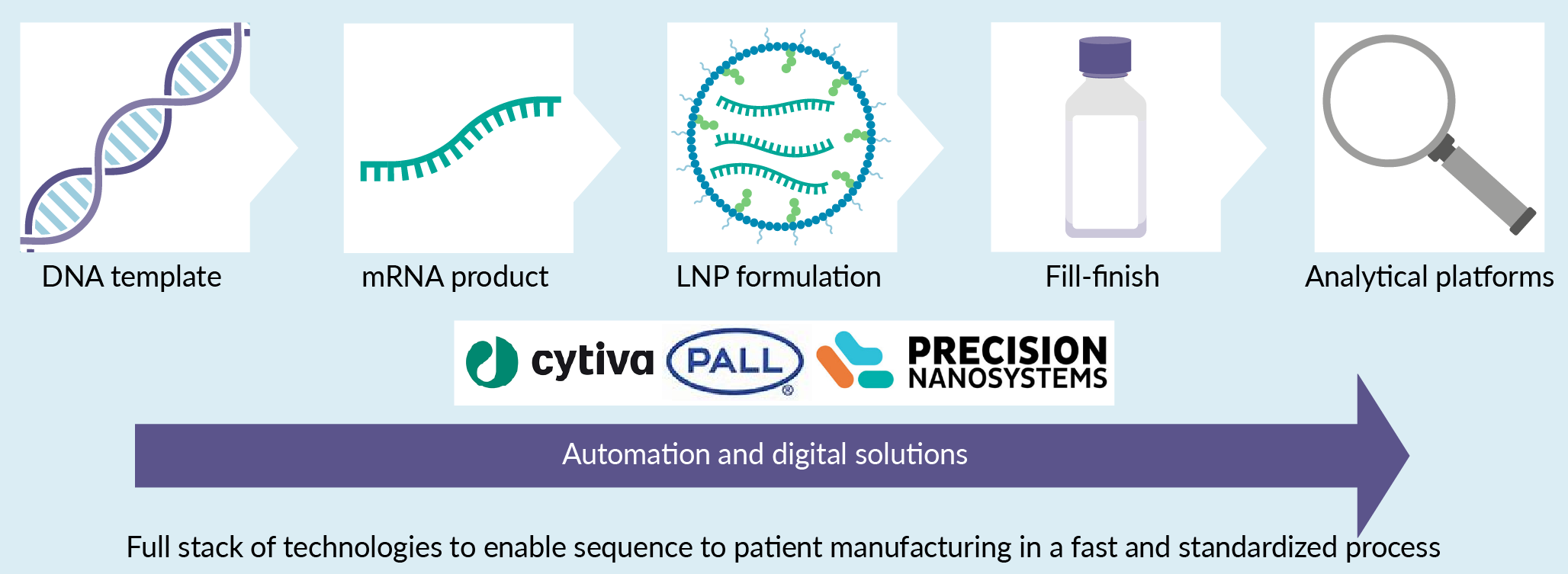
The successful development and rapid deployment of the messenger RNA (mRNA) vaccines against SARS-CoV-2 virus during the COVID-19 pandemic has catalyzed the industry to look even more closely at the technology beyond their potential use for novel vaccines to enable breakthrough treatments for cancer, rare diseases and more. Indeed, the mRNA and lipid nanoparticles (LNP) technologies that underpin the COVID-19 vaccines have far-reaching potential to transform modern medicine. However, as a relatively new technology, there remain barriers to successful industrialized manufacture of LNP-encapsulated mRNAs (mRNA–LNPs).The manufacturing of the mRNA–LNP drug product can be broken down into five key steps (see figure below): DNA template manufacturing, mRNA drug substance synthesis and purification, mRNA–LNP formulation and purification, fill/finish operations, and analytical testing. This article will first examine each step and discuss challenges and opportunities pertaining to the process itself and for the manufacturing facilities.