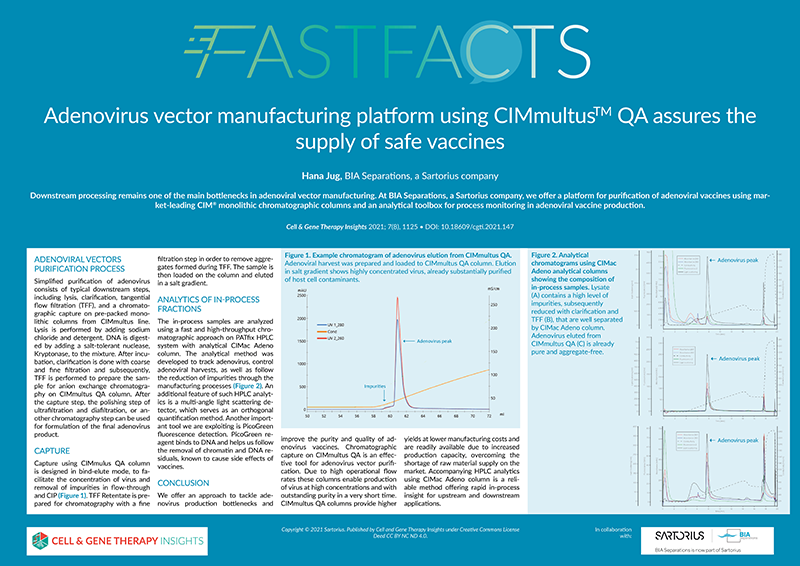
Adenovirus vector manufacturing platform using CIMmultus™️ QA assures the supply of safe vaccines
Cell & Gene Therapy Insights 2021; 7(8), 1125
10.18609/cgti.2021.147
Watch the video or read the poster to learn:
- Why downstream processing remains one of the main bottlenecks in adenoviral vector manufacturing
- How an adenoviral vector purification platform using CIMmultus™ QA as the key purification step, secures a fast and robust process with better purity
- CIMmultus™QA offers high capacity and high yields of adenovirus, results in cost reduction and overcomes raw material supply bottlenecks
We hope you enjoyed this FastFacts video. You can also view the summary here.
Authorship & Conflict of Interest
Contributions: All named authors take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Acknowledgements: The author would like to thank Katja Vrabec, Ana Mavri and Maja Leskovec for their help with this FastFacts article.
Disclosure and potential conflicts of interest: H Jug is an employee of BIA Separations, a Sartorius Company. The author has no other conflicts of interest to disclose.
Funding declaration: The authors received no financial support for the research, authorship and/or publication of this article.
Article & copyright information
Copyright: Published by Cell and Gene Therapy Insights under Creative Commons License Deed CC BY NC ND 4.0 which allows anyone to copy, distribute, and transmit the article provided it is properly attributed in the manner specified below. No commercial use without permission.
Attribution: Copyright © 2021 BIA Separations, now a Sartorius company. Published by Cell and Gene Therapy Insights under Creative Commons License Deed CC BY NC ND 4.0.
Article source: From a FastFacts video recorded on: Sep 17 2021; Publication date: Oct 05 2021.