Applying a closed, modular, semi-automated system to CAR T cell therapy manufacturing
Cell & Gene Therapy Insights 2021; 7(8), 961–972
10.18609/cgti.2021.127
There are many benefits of implementing closed, GMP-compliant, digitally connected solutions at earlier stages in CAR T cell therapy development. In this article, we give an overview of one such modular system, followed by a panel discussion with cell therapy industry experts on overcoming bottlenecks in CAR T cell manufacturing with automated solutions.
Due to the novelty of the cell therapy industry, and the inherent living cell characteristics of the product, the field is facing a lot of challenges – from supply chain, to logistics, to bioprocessing. How do we scale up and scale out? How do we manage the quality and performance of the product? All of these challenges ultimately boil down to manufacturing; as the saying goes, the process is the product.
For autologous T cell therapy, specifically, the process typically involves a lot of hands-on, labor-intensive work with a lot of open processes, complexity, and different products. Plus, the instruments applied in one process are normally not flexible to be used in other processes.
We believe closed, modular, and automated systems will help to overcome some of these challenges, and ultimately get therapies to patients faster. Thermo Fisher Scientific has the largest collection of reagents and protocols of any vendor, a number of existing cell processing platforms, a range of analytical and in-process tests, and sophisticated digital science tools – by combining these capabilities, we have the ability to integrate the whole process into one workflow to better serve the cell therapy industry.
With this goal in mind, we created a series of modules or unit operations, each of which can operate independently of each other. A software library connected to the central database system can connect these individual modules into a whole workflow solution.
PBMC isolation
As shown in Figure 1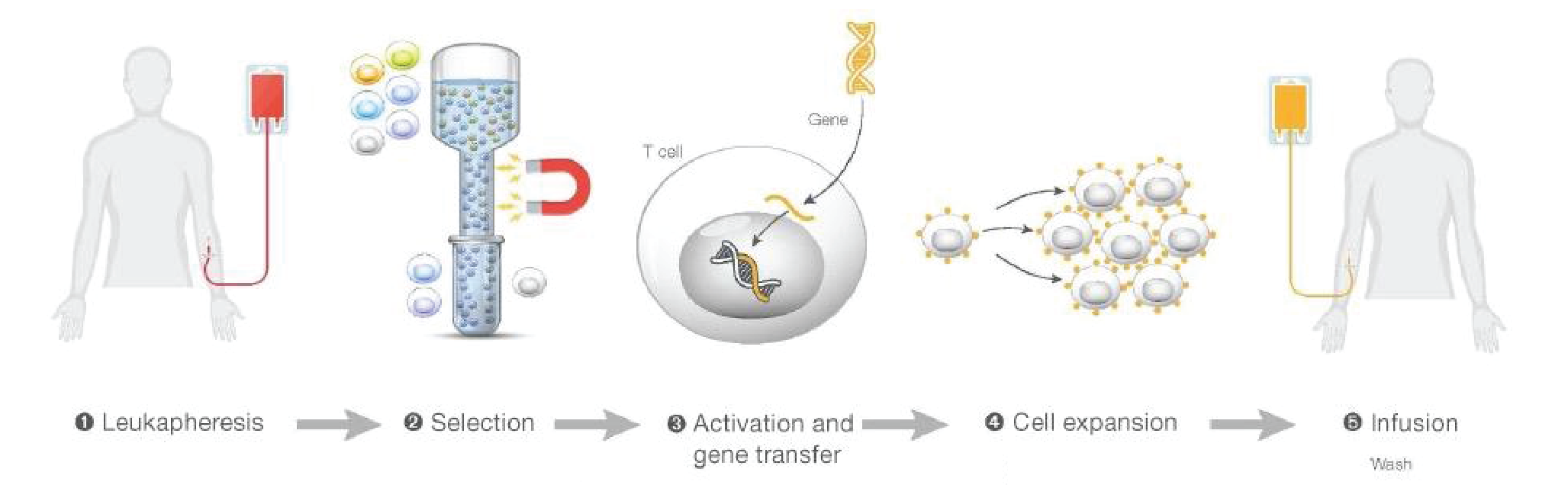
To solve this problem, we developed the Gibco™ CTS™ Rotea™ System – a closed and automated system with a small footprint and its own single-use consumables. The underlying principle is counterflow centrifugation which means we introduce a flow force that counterbalances the G force. Once the flow force and G force equilibrate, the cells are floating in the chamber, and create a fluidized cell bed. Cell populations with different sizes and buoyancies can be easily separated. Figure 2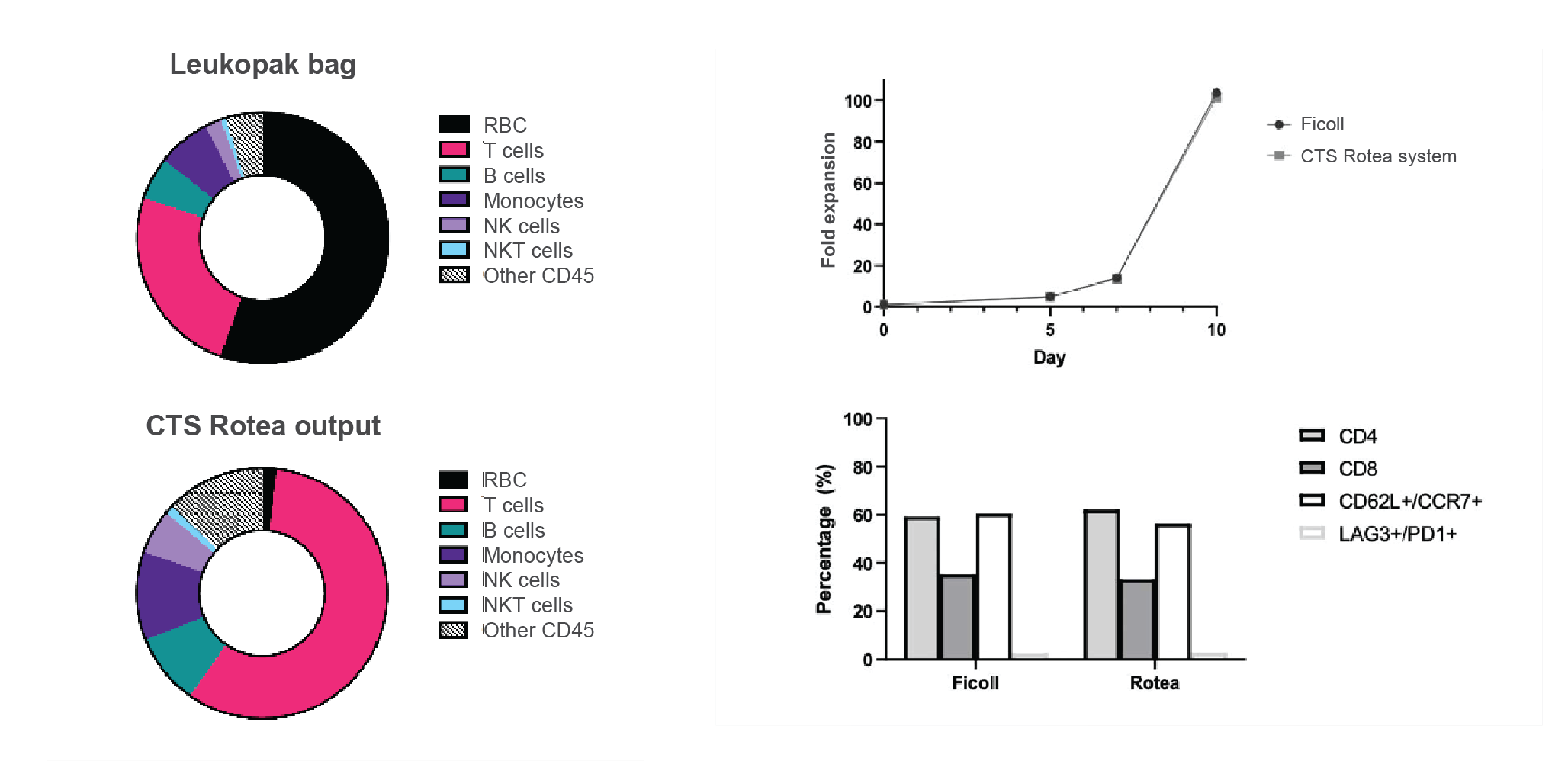
T cell selection & activation
The next step is T cell selection, for which we use CTS Dynabeads™ CD3/CD28, in combination with a DynaMag™ magnet. The benefit of the Dynabead system is that it not only selects the T cells but also activates them. Figure 3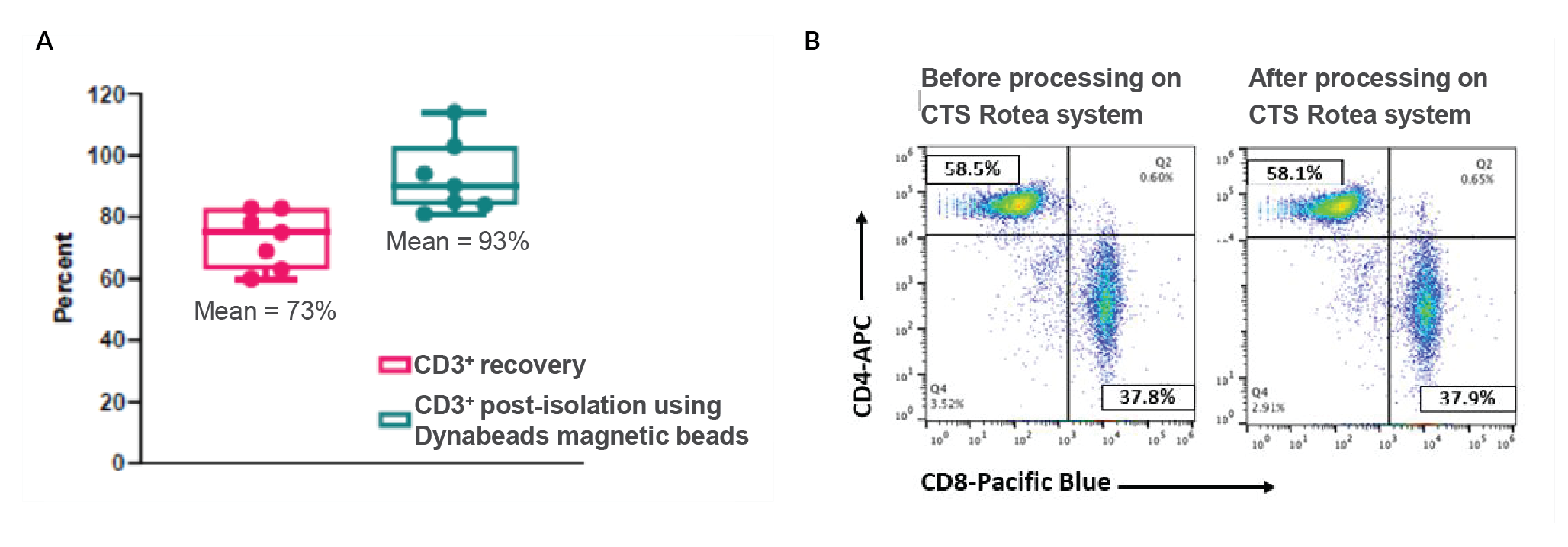
We have developed an all-in-one process to combine PBMC isolation and T cell selection/activation in one step, using Rotea (Figure 4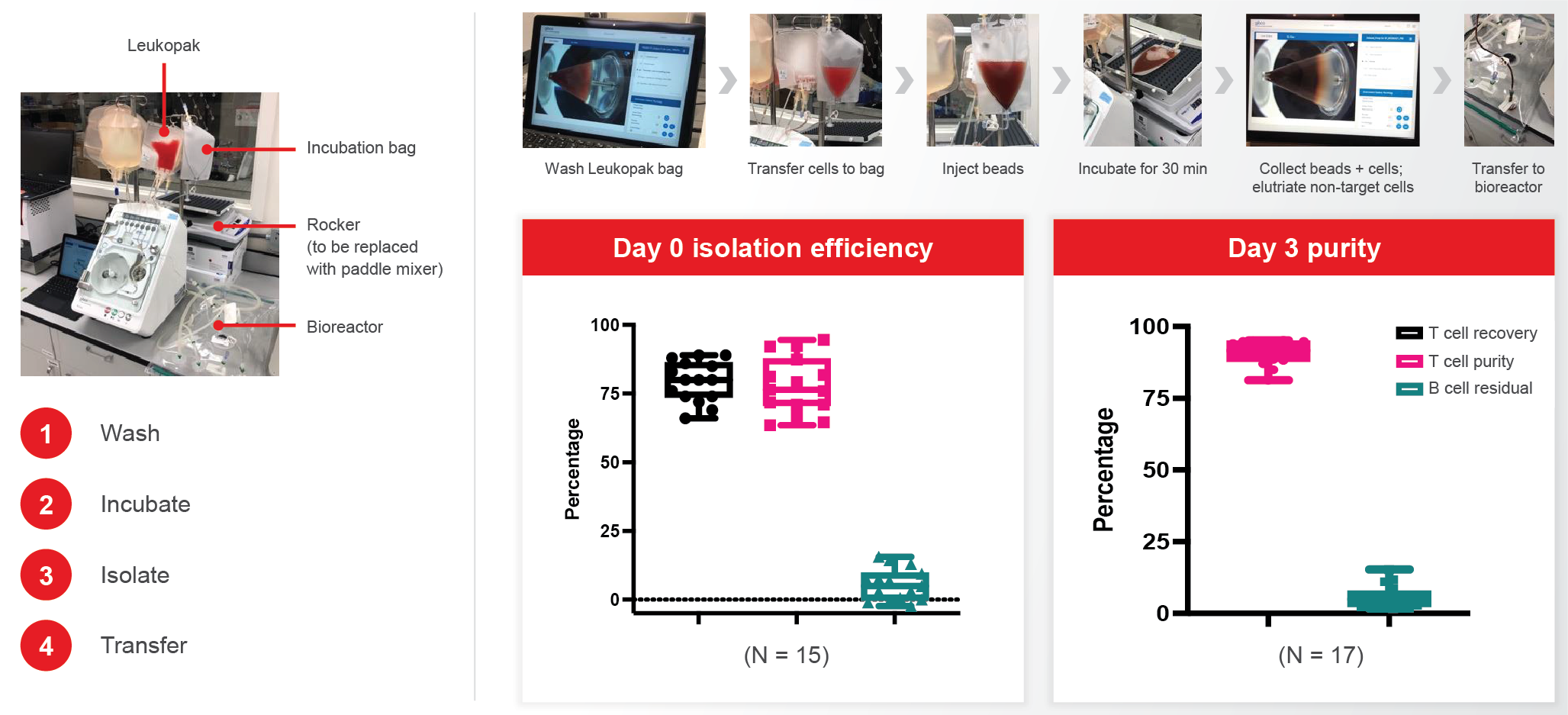
Electroporation
The next step is to wash and concentrate the cells and carry out buffer exchange before electroporation. This step and the harvest and formulation steps further downstream all use Rotea. Typically, 86% cell recovery can be achieved in this step.
Next comes electroporation to introduce CAR genes into the T cells. Thermo Fisher Scientific has two electroporation systems available: Invitrogen™ Neon™ Electroporation System is designed for research use, R&D, or small-scale screening, whereas the large-volume electroporation Gibco CTS Xenon™ system can host a 1 ml single shot or 5–25 ml flow-through shot (Figure 5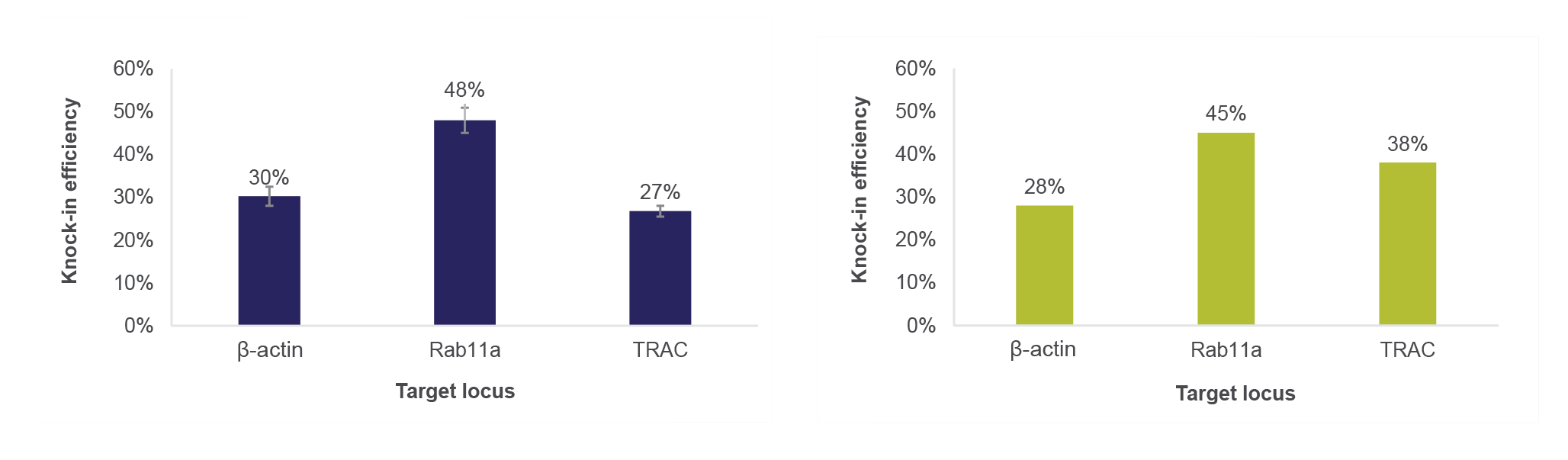
We are actively working on connecting the pre-electroporation wash, concentrate, and buffer exchange steps (using Rotea) with electroporation using Xenon, in order to achieve automation. A prototype system can be seen in Figure 6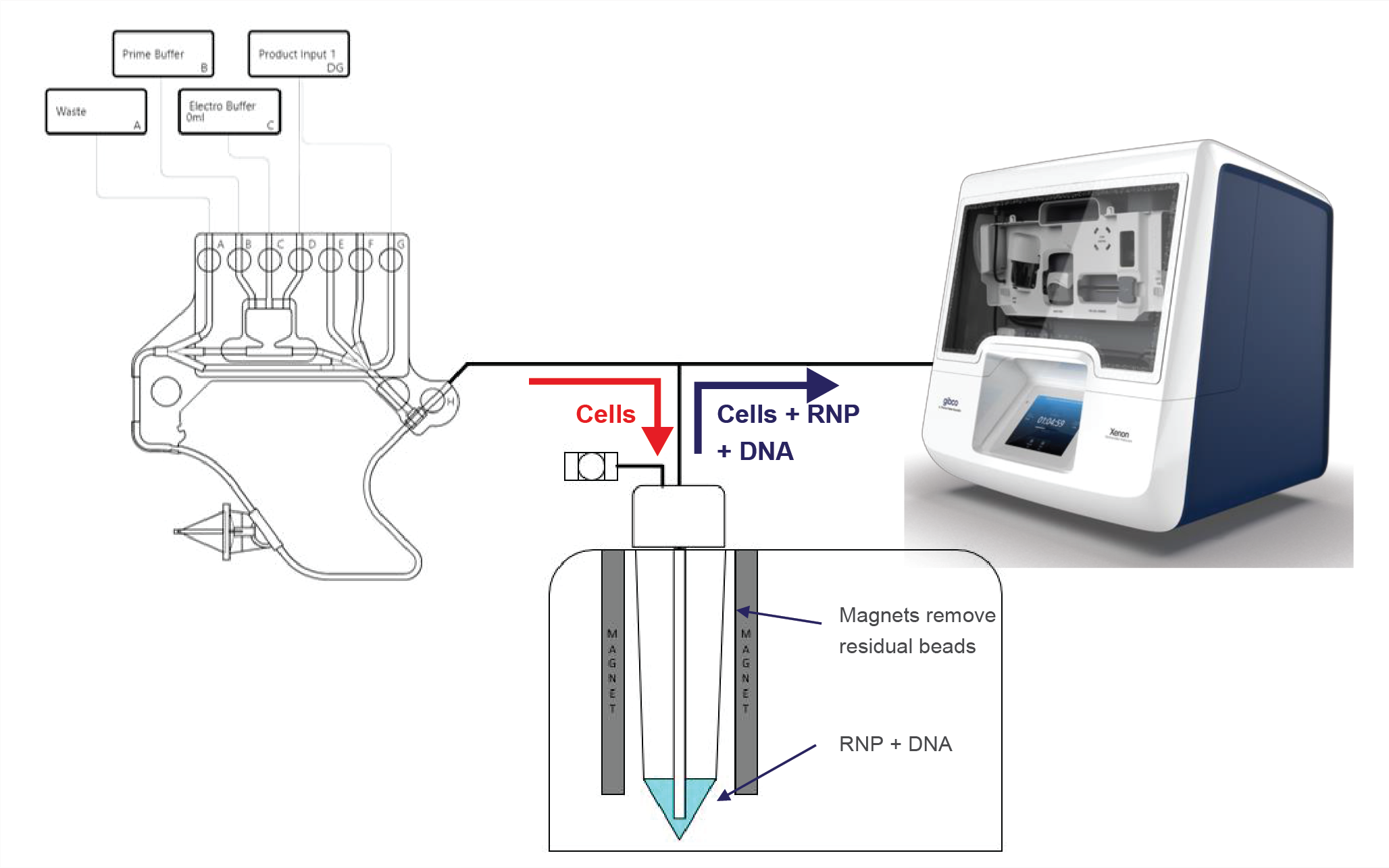
Expansion
To expand the newly created CAR T cells, Thermo Fisher Scientific supplies Gibco CTS OpTmizer™
T Cell Expansion Serum-Free Medium (SFM) and Gibco CTS Immune Cell Serum Replacement (SR), which are proven to provide very good T cell expansion and high viability. More importantly, this combination promotes enrichment of the T cell memory phenotype and a balanced CD4:CD8 ratio.
The HyPerforma™ rocker system can further facilitate robust T cell expansion. In combination with our T cell media, expansion is comparable to competitor perfusion systems and outperforms static systems (Figure 7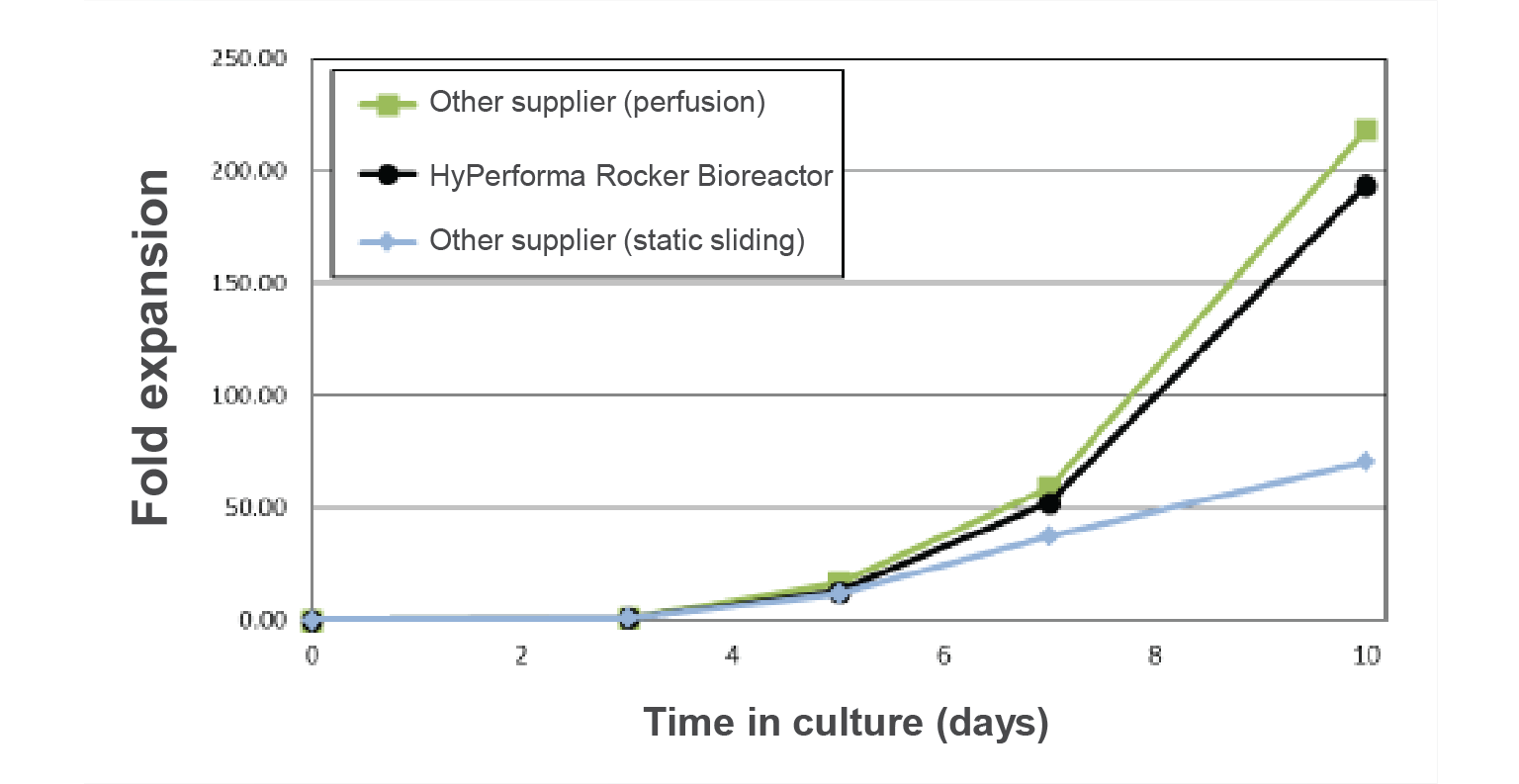
Cryopreservation
After cell expansion, there is another round of buffer exchange, formulation, and harvest, before the final step – cryopreserving the CAR T cells for shipping back to hospitals. Thermo Fisher Scientific’s controlled rate freezer, the CryoMed™, has been extensively used in the cell therapy industry. It provides repeatable and consistent temperature performance for the final product and allows the use of different consumables. The freezer has preset programs for T cells, or the user can set their own protocols. Cell viability after thaw is around 90%.
By combining these different solutions, a complete, closed workflow solution for non-viral CAR T manufacturing can be achieved. The next step for Thermo Fisher Scientific is to integrate all of these modules into the system so that we can achieve true automation.
Panel Discussion
Yongchang Ji, Semsi Ensari, and Nathan Moore answer your questions on bottlenecks in CAR T cell manufacturing and how automate solutions can help achieve better modularity, standardization, compatibility, and scalability.
Q What are the key critical bottlenecks and challenges in CAR T cell therapy manufacturing, and how have these evolved over recent times?
SE: The three things that keep me awake at night are:
- Supply-demand challenges;
- Logistics related to the supply chain; and
- Automated technology versus manual processing.
In terms of supply and demand, companies have to consider the capacity of each production facility. For example, based on that capacity, how much demand can they accommodate at any time? For autologous therapies, everything must be prepared in accordance with where the demand is and the disease area you are dealing with, and that becomes a big challenge for capacity at the manufacturing site.
Looking at logistics and supply chain, factors such as disease state and stress conditions of the cells play a major role in bringing the cells from the treatment center into the facility and from the facility back to the patient. This is a huge challenge when you’re dealing with only one patient at a time.
Last but not least is the technology. What systems can you utilize to separate the cells, expand them, and freeze them to deliver back to the patient, versus preparing these things in a manual setting?
These three topics are all connected. If you’re going from manual to newer technology, you’re also enabling larger supply because you’re able to process faster and utilize your capacity better.
NM: A lot of the big challenges are around the scalability of these technologies – going from manual processes into larger processes, and incorporation of automation. That’s one of the big bottlenecks.
YJ: I agree with both Semi and Nathan that turnaround time is a key factor to consider. I also want to talk about standardization. Given the autologous nature of the product, how can we as an industry establish a standard assay (for example, how to assess the purity)? Is 80% purity good enough, or do we want to achieve even higher? And what kind of residual contamination is acceptable? Those are things we need to take into consideration.
Q What would you define as the key benefits in switching to a semi-automated manufacturing platform in this sector?
NM: The obvious benefits are reducing labor and increasing control, which will hopefully give you a more consistent process, and ultimately a more consistent product.
Another big advantage is the ability of well-designed automation technologies to close your process and reduce the risk of contamination, which is high with manual processes where you are going in and out of plates or bags.
The last piece, and something that is often overlooked, is the ability of automated systems to provide more detailed data and process logging so you’re capturing much more information about your system and process overall – and risks or deviations are more clearly laid out.
YJ: By using modular technology, you are also creating consistency in processing the cells and making training easier. Each of the machines can be used in multiple steps so you only need to train on that machine once.
SE: A high-demand, semi-automated process gives us a huge advantage. However, with any kind of advantage, there comes a challenge, and one challenge is maintaining a sustained supply for both the equipment and the disposable kit.
Q What do you see as the key considerations to bear in mind when you’re looking to make the switch to a more automated process? What issues can arise and how can you work to alleviate or address them ahead of time?
YJ: I would say consistency. We want automation, but we don’t want to compromise the product quality. For example, in the modular approach, how can we set the criteria for each of the module operations? Plus, how do we consider this module when placed in the workflow? How does the upstream workflow affect the downstream?
I’ll give you an example from our work on electroporation. When we tried to connect the steps into a workflow, we found that different vessels have a dramatic impact on the electroporation knock-in efficiency. We’re now trying to figure out what is affecting the knock-in efficiency at the transcription level.
Another concern people have around modular automation is what happens if the equipment malfunctions. We need a set of recovery protocols ready because losing a patient sample is not an option. We also need to think about the future – how we can integrate the different platforms if the process changes?
SE: For me, a key consideration is the ability to meet different expectations from the system – what is it designed for? What are the minimum and maximum volumes for processing and what type of cell range can it maintain? Similarly, how can we make it more reliable? We also need good feasibility studies in relation to the technology before moving into more process development.
NM: The other panelists touched on it, but it’s important to understand what your key performance criteria are at the end of the process. Plus, considering the end-scale or end-manufacturing process you’re looking at, and making sure your selected tool is capable of meeting your full-scale capabilities. Before you start the development, you need to have a clear sense of what the key metrics of success are for that tool.
Q How important are tools that work across multiple platforms and processes?
SE: Cell and gene therapy includes many disease forms and mechanisms of action. Automation tools should help us with different immune cell interactions and, more importantly, how we seamlessly integrate each unit operation all the way from isolation to formulation. Plus, there’s process/product monitoring – how do we maintain a system of operations that can integrate these things in a very coherent way?
NM: I think the flexibility offered by such systems is key. Particularly within a process, but across processes as well. You also have to think of limitations such as space in GMP suites and manufacturing facilities, where you don’t want to have 13 different tools if you can avoid it. If you have one tool that can work early on in your process at a smaller scale, and later in your process at a larger scale, that adds a lot of value to that tool. In your manufacturing suite, you may rotate through multiple processes over time – you don’t want to decommission half of your manufacturing suite to bring in new tools if you can avoid it.
YJ: In addition to what has already been said, it also reduces the need to do multiple qualifications of the machine. Plus, it helps with training – if you have the same machine that is used across different platforms, staff are already familiar with it. In the presentation, I already gave the example of the Rotea, which can be used for electroporation buffer exchange earlier in the process, as well as wash, concentrate, and formulation later on. These two protocols are very similar, and the operator can adapt one protocol to another on these different modules. We designed it to be an open platform so people can adapt their own process using the machine.
Q It certainly looks like there are some good options for each unit operation. When do you think a fully integrated CAR T production system might realistically be available?
SE: Everyone is trying to make it happen! There are some systems in development, but the question is how widely these will be available so that everyone can utilize them. And, more importantly, can the processes that have already been commercialized fit the available tools? At Kite, we are working with suppliers to make a fully automated system available. But I think it will be at least a couple more years to see such advances.
Q What are the best ways to quantify the performance of cells with critical quality attributes (CQAs) to make decisions on optimizing the cell manufacturing process, and can these tests be integrated into closed processes?
NM: It’s a great – and complex – question. You must have clear metrics of the functionality of your cells, independent of your process. The specific CQAs will vary depending on what type of cell or gene therapy you are producing. As to whether those tests can be integrated into closed processes, I certainly would like to see that, but it’s difficult. Depending on what the assay is you may be able to incorporate some of those into these processes, particularly if they’re protein-based, as a functional readout.
Q Is starting material variability typically a challenge to the processes?
YJ: It is a real challenge, in our experience. We’re dealing with healthy donors but, even then, the content of the product varies so much. Some people may have 20% CD3, while others may have 40%.
NM: I agree. It is important to map things out carefully. Again, defining success criteria for a technology and then evaluating multiple samples in your process development to make sure that, despite any variation that you see, the process is still going to meet your success criteria.
Q How do you balance flexibility to enable process development and support a range of cell types versus wanting to move to commercial-scale manufacture and minimize your capital expenditure?
SE: As other panelists have noted, it’s important to test the system with various cells during process development. The use of clinical subject material during development will give a lot of opportunities to design the process to meet the needs of different patient populations as well. Without the patient material, it becomes very difficult to design a process that could accommodate various patients. As we discussed earlier, patient cells are not easily replaceable so having a highly reliable commercial unit is a high priority.
Q Could you share your vision for the further evolution of tools and processes for CAR T cell therapy manufacture?
NM: I think the future is being able to hook up these modular systems together directly. Or having robotic systems that can move your bag between systems. Sitting on top of this is integration with analytical tools that can be taking real-time measurements, and ultimately having this all controlled through predictive modeling and AI. That removes a lot of human error from the process.
SE: I agree with Nathan that seamless integration with each unit operation is a key goal. The technology should also accommodate scale-up of manufacturing with high quality, and meet the need for high-demand scenarios. Keeping cost of goods low is also key for cell therapy manufacture. Manual processes are highly costly, which limits availability for patients.
YJ: I think two pathways are evolving for autologous, as the CAR design gets more efficient, so there will be more decentralized work – maybe all-in-one or even microfluidic tools can be developed for the manufacturing of autologous CAR T. Allogeneic CAR T cell production tends to be more centralized, and we need to figure out a way to scale up the manufacturing so that the product is ready for use whenever patients need it.
Authorship & Conflict of Interest
Contributions: All named authors take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Acknowledgements: None.
Disclosure and potential conflicts of interest: Y Ji is an employee of Thermo Fisher Scientific. N Moore declares stock options in Takeda Pharmaceuticals. He also has patents with Draker and Takeda Pharmaceuticals for multiple cell bioprocessing tools. The authors declare that they have no other conflicts of interest.
Funding declaration: The authors received no financial support for the research, authorship and/or publication of this article.
Article & copyright information
Copyright: Published by Cell and Gene Therapy Insights under Creative Commons License Deed CC BY NC ND 4.0 which allows anyone to copy, distribute, and transmit the article provided it is properly attributed in the manner specified below. No commercial use without permission.
Attribution: Copyright © 2021 Thermo Fisher Scientific. Published by Cell and Gene Therapy Insights under Creative Commons License Deed CC BY NC ND 4.0.
Article source: This article is a transcript of a previously published webinar, which can be found here.
Webinar published: Jun 29 2021; Publication date: Sep 14 2021.