De-risking the final formulation, fill and finish step in cell therapy manufacturing: considerations for an automated solution
Cell & Gene Therapy Insights 2020; 6(10), 1513–1519
10.18609/cgti.2020.165
Final formulation and cryopreservation of the cell product is a critical and high-value step in cell therapy manufacturing. At this stage, the cells have been through a tedious selection, modification and expansion process, and maintaining their health and viability through this step is of utmost importance. This downstream step of final formulation heavily relies on manual processes that bring inherent user-dependent variability, and it becomes unsustainable with the demand for increased output in a manufacturing setting. The necessity to reduce open process steps, ensure process traceability, minimize DMSO contact time with cells and facilitate electronic data capture establishes the need for flexible automation at this step. The preferred automated system should be functionally closed with single-use disposables and should provide the ability to scale the process in a reproducible fashion. The system should reduce the number of open events compared to the current manual process and ensure material and process data traceability using industry-accepted computation. Implementing automation at this step not only ensures a more consistent product, it may also be an effective way to reduce risk at a critical step to support product delivery to the patients, while also reducing manufacturing costs.
Regenerative Medicine (RM), a branch of medicine that regrows, repairs or replaces damaged cells or tissues, has become an exciting and groundbreaking scientific field. As of August 2020, there were 1,078 active clinical trials in the field for multiple disease indications. Out of over 1,000 companies in the RM space, half are cell and gene therapy companies [1]Alliance for Regenerative Medicine (ARM). Innovation in the Time of COVID-19: H12020 ARM Global Regenerative Medicine and Advanced Therapy Sector Report. August 2020: https://alliancerm.org/sector-report/h1-2020-report-pdfAlliance for Regenerative Medicine (ARM). Innovation in the Time of COVID-19: H12020 ARM Global Regenerative Medicine and Advanced Therapy Sector Report. August 2020: https://alliancerm.org/sector-report/h1-2020-report-pdf. Given the innovation boom and clinical pipeline in the cell and gene therapy space, the US Food and Drug Administration (FDA) expects to receive hundreds of new investigational new drug (IND) applications by 2025 and to approve 10 to 20 cell and gene products per year [2]Statement from FDA Commissioner Scott Gottlieb, MD, and Peter Marks, MD, PhD, Director of the Center for Biologics Evaluation and Research, on new policies to advance development of safe and effective cell and gene therapies. January 2019: https://www.fda.gov/news-events/press-announcements/statement-fda-commissioner-scott-gottlieb-md-and-peter-marks-md-phd-director-center-biologics. In the first half of 2020, a record-setting $10.7 billion was raised for therapy development, manufacturing and clinical infrastructure [1]Alliance for Regenerative Medicine (ARM). Innovation in the Time of COVID-19: H12020 ARM Global Regenerative Medicine and Advanced Therapy Sector Report. August 2020: https://alliancerm.org/sector-report/h1-2020-report-pdfAlliance for Regenerative Medicine (ARM). Innovation in the Time of COVID-19: H12020 ARM Global Regenerative Medicine and Advanced Therapy Sector Report. August 2020: https://alliancerm.org/sector-report/h1-2020-report-pdf.
One of the most exciting and evolving branches in the cell therapy space is autologous adoptive immunotherapy, which requires acquisition of a patient’s immune cells by apheresis followed by selection of the target cell type, genetic modification, expansion and final formulation before reinfusion into the patient for the desired therapeutic effect. The exciting era of ‘living drugs’ brings its own complexity and challenges. One of the challenges is to manage the logistical transport of cells among multiple facilities, making cryopreservation a necessity for the large-scale commercial manufacturing process [3]Panch SR, Srivastava SK, Elavia N et al. Effect of cryopreservation on autologous chimeric antigen receptor T cell characteristics. Mol. Ther. 2019; 27(7): 1275.. Final formulation and cryopreservation of the cell product to prepare it for transfusion is potentially the most critical step in manufacturing. At this stage, the cells have been through a tedious selection, modification and expansion process, and maintaining their health and viability through this step is all-important. Attention should be given to the formulation, fill and finish step during process development in order to fully understand the impact of formulation and cryopreservation on manufacturing and the cellular end product.
Downstream processing: DMSO in final formulation, fill & finish
One of the key steps of downstream processing is the addition of cryoprotectant to a cellular suspension. Cryoprotectants, such as dimethyl sulfoxide (DMSO), are chemicals that prevent cells from damage during freezing. A recent review by Awan et al. summarizes the role of DMSO in cryopreservation and its toxic effects for both in vitro and in vivo uses [4]Awan M, Buriak I, Fleck R et al. Dimethyl sulfoxide: a central player since the dawn of cryobiology, is efficacy balanced by toxicity? Regen. Med. 2020; 15(3): 1463.Awan M, Buriak I, Fleck R et al. Dimethyl sulfoxide: a central player since the dawn of cryobiology, is efficacy balanced by toxicity? Regen. Med. 2020; 15(3): 1463.Awan M, Buriak I, Fleck R et al. Dimethyl sulfoxide: a central player since the dawn of cryobiology, is efficacy balanced by toxicity? Regen. Med. 2020; 15(3): 1463.. The authors highlighted the critical place of DMSO in cryobiology and its widespread use in the field. Figure 1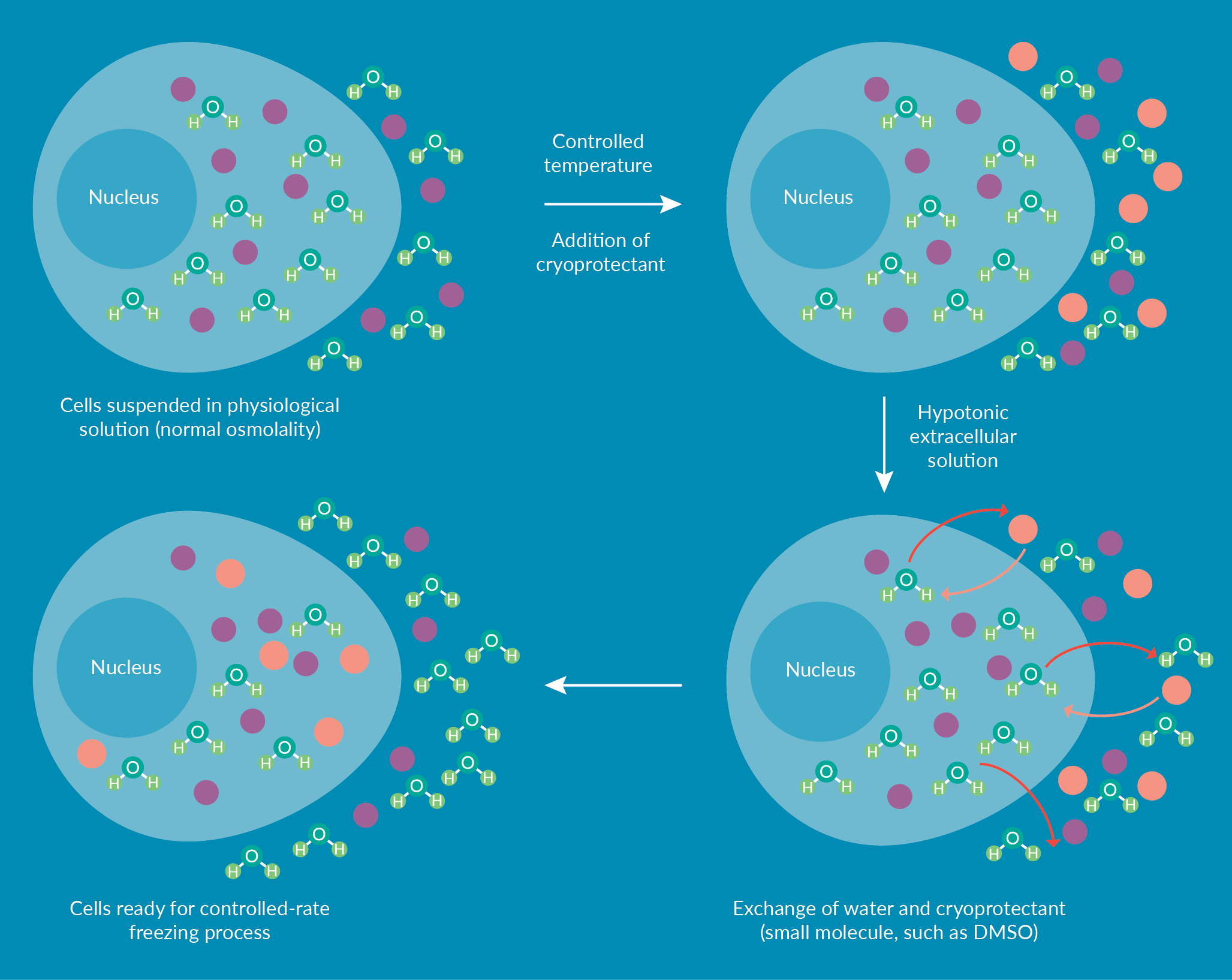
Risks of a manual formulation, fill & finish process
With new regulatory approvals, potentially curative cell therapies are reaching more patients globally and the need for greater manufacturing scale is increasing in parallel. While automation is being adopted at multiple steps of the manufacturing process, the downstream step of final formulation, fill and finish still often relies heavily on manual methods. Manual methods, however, lack the process controls at this step that may impact the viability and functionality of cells at thaw. Any open, manual step in the process is heavily susceptible to contamination and operator-to-operator variability [8]Iyer RK, Bowles PA, Kim H, Dulgar-Tulloch A. Industrializing autologous adoptive immunotherapies: manufacturing advances and challenges. Front. Med. 2018; 5: 150. .
Although a manual process can be used in a manufacturing setting, there are inherent risks that could potentially affect a patient. Open events during washing, dilution, and cryoprotectant addition are subject to contamination. Another risk during a manual process is the potential for human error. The clinical dose administered to the patient relies on accurate cell counting and volumes, so something as simple as misreading a measurement or transposing numbers during data capture could be detrimental. Final formulation is a key step in cell therapy manufacturing. Loss of the cell product at this stage due to human error would be costly not only in financial terms; the patient awaiting a potentially lifesaving cellular therapy would not receive it.
Mishandling of the cells by premature addition of cryoprotectant media, a heterogenous mixture with an imbalanced ratio of cryoprotectant to cell volume, or a lengthy exposure of the cells to DMSO could result in decreased health or viability of the cell product. As previously mentioned, DMSO is an organic solvent, and its mixing with aqueous solutions, such as cell culture media, is exothermic. The risk of heat damage to cells is reduced by pre-cooling the cryoprotectant and slowly adding it to the cells. Maintaining the product bags between gel packs is a key step during the manual addition of the cryoprotectant, but there is a general lack of process controls such as temperature set point and tolerance limits of the temperature for the bag. Syringe pumps may be used to control the speed of cryoprotectant addition but do not ensure product uniformity in each bag. Although these mitigations provide reasonable assurance, they are not standardized among users and institutions. As with any manual process, there are chances of operator-to-operator variability, and even a single technologist may inadvertently not treat every product bag in the same way. This may result in incorrect volume ratios or non-homogenous mixtures.
Accurate documentation of the steps is extremely important in the manufacturing process, but manual methods are error-prone and may result in missing data, a lack of material workstream control, and too much total exposure time of cells to DMSO. Manual documentation can slow down the process, resulting in longer-than-necessary DMSO exposure and process times. Manual methods also rely on additional personnel; quality oversight of manufacturing operations can be a major time and cost expense in manufacturing platforms [9]Ball O, Robinson S, Bure K, Brindley DA, McCall D. Bioprocessing automation in cell therapy manufacturing: outcomes of special interest group automation workshop. Cytotherapy 2018; 20: 592.. Staff costs can also be one of the largest operational costs due to high training costs and production staff turnover [10]Harrison RP, Medcalf N, Rafiq QA. Cell therapy-processing economics: small-scale micro factories as a stepping-stone toward large-scale macro factories. Regen. Med. 2018; 13(2): 159..
The risks and costs associated with a manual fill and finish process, however, can be reduced by switching to automation. Automation reduces the risk of human error, controls the addition of cryoprotectant, monitors temperature set points and tolerance limits, and mixes each product consistently. Automation also reduces the need for additional quality control (QC) personnel to oversee the process, thus creating more reliability while reducing labor costs.
Considerations for a functionally closed, automated system
When considering a functionally closed system that can enable automation of formulation, fill and finish, there are several important factors to consider. First and foremost, any automation strategy should utilize functionally closed systems to reduce the number of open steps in the process. The cell therapy products cannot be sterilized at the end of manufacturing; therefore, it is critical to ensure their sterility through aseptic processing [11]Levinson Y, Eylon Y, Heymann A, Zaretsky-Rits A, Karnieli O. Foundation elements for cell therapy smart scaling. BioProcess Int. 2015; 13(4)s.. A functionally closed system can maintain the integrity of the process and considerably reduces the risk of contamination. The system should work with single-use disposables that can facilitate the introduction of materials, mixing of the combined products for homogeneity and aseptic filling of product bags. It should also facilitate automated mixing of the products to ensure consistent and homogenous product between bags. The process must also automatically and consistently remove air within the functionally closed system without compromising the volume of cell product. The automated device should deliver final product bags closed with a single dependable seal to avoid introducing additional process risks.
In addition, the automated system should allow process controls for temperature and the rate of cryoprotectant addition. Reiterating the point, the addition of cryoprotectant such as DMSO, to a cell suspension is an exothermic reaction and the release of energy could damage the cells [6]Baboo J, Kilbride P, Delahaye M et al. The impact of varying cooling and thawing rates on the quality of cryopreserved human peripheral blood T cells. Nature 2019; 9: 3417.Baboo J, Kilbride P, Delahaye M et al. The impact of varying cooling and thawing rates on the quality of cryopreserved human peripheral blood T cells. Nature 2019; 9: 3417., [12]Hornberger K, Yu G, McKenna D, Hubel A. Cryopreservation of hematopoietic stem cells: emerging assays, cryoprotectant agents, and technology to improve outcomes. Transfus. Med. Hemother. 2019; 46: 188.. Considering this significant need for process controls and slow addition of pre-cooled cryoprotectant, an automated system must provide controlled-rate addition of cryoprotectant and should monitor the temperature throughout the formulation and fill process.
Another important consideration for an automated system is that it should provide traceability of the formulation, fill and finish steps to facilitate Good Manufacturing Practices (GMP) compliance. The system should include electronic data capture that facilitates compliance with CFR Part 11, including data encryption, data maintenance and transfer within the system. User authentication, or password-protected login, is needed for device security and workflow or configuration management. Data recording of the process, including process alarms or flags based on user-controlled limits and tolerances, as well as the ability to pull data from the system, are also critical. The process would also benefit from a barcoding system that enforces the order of operations by validating each barcode before executing the associated process step.
The final consideration for an automated fill and finish system is the ability to scale the process in a flexible way that can be adjusted to the therapeutic need or the need to meet treatment demands. This includes allowing the user to choose the number of cryobags and the volumes that are needed in each.
Data snapshot of an automated and a manual process
The Finia® Fill and Finish System is an example of an automated and flexible platform that offers electronic data capture and helps facilitate compliance with current GMP regulations. The system allows cryopreservation in single-use disposable sets with cryopreservation-friendly product bags. The disposable set, offered in two different volume configurations, contains three product bags plus a quality control bag for post-formulation testing. On the device, the chiller plate and mixing arm ensure the cell suspension and cryoprotectant media are mixed in the appropriate volumes, are maintained at the set temperature within tolerance limits and remain homogenous before being aliquoted into the product bags at the set volumes.
In an experimental comparison, outlined briefly in Figure 2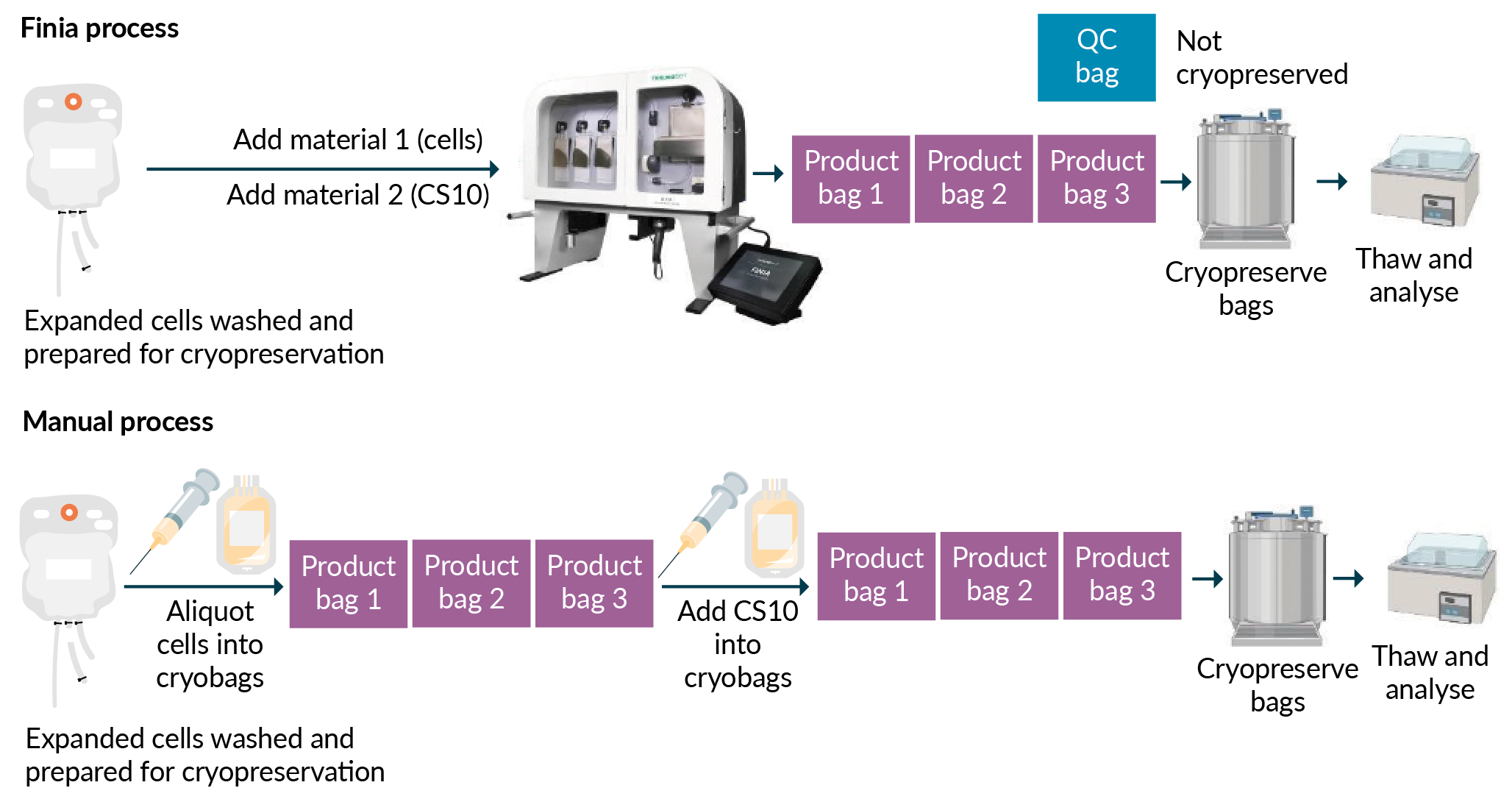
Using 1:1 dilution of cells to CryoStor®CS10 (BioLife Solution), the cells were cryopreserved at a final concentration of 10 × 106 to 12 × 106 cells per mL, in a final DMSO concentration of 5%. The cells were then placed in a controlled-rate freezer (CRF) and the temperature was dropped 1°C per minute until the product temperature reached -100°C. The product bags were then placed in a liquid nitrogen freezer for at least 72 hours. At the time of thaw, the product bags were placed in a 37°C water bath until a small portion of ice remained. The thawed product bags were taken into a biosafety cabinet to pull a sample for cell count and viability. Cell count and viability were measured by trypan blue exclusion using Vi-CELL™ XR (Beckman Coulter). The results showed that expanded healthy CD3+ T cells formulated using either Finia® or the manual method maintained greater than 90% cell viability post-thaw. The post-thaw cells were then diluted 1:1 in Cell Thawing Media 10% Dextran 40 (in 0.9% NaCl; BioLife Solutions), washed using centrifugation, and resuspended in complete media. The cells were expanded post-thaw for 48 hours with cell counts and viability measured daily. Mean viability for post-thaw samples formulated and filled using Finia® remained above 90% for up to 48 hours in culture, compared to above 89% for samples processed manually [13]Sethi D, Cunningham A, Massey K. Data-driven insights on cell therapy fill and finish processes. Genetic engineering & biotechnology news (webinar). July 23, 2020: https://www.genengnews.com/resources/webinars/data-driven-insights-on-cell-therapy-fill-and-finish-processes/.
Conclusion
For cell-based therapies, formulation, fill and finish is an important element in the manufacturing process. As the final step prior to reinfusing patients in the hospital, it is critical that the integrity of the final cell product remain intact. Workflow controls and electronic data capture are key factors to ensure a consistent process. Automation, coupled with functionally closed systems, is a way to ensure the cellular product is viable, consistent between product bags, and traceable from a GMP perspective. Automation can also drive down labor and facility costs and maintain reliable processes with electronic records of each action performed. For autologous cell therapies, an automated fill and finish process should be considered.
References
1. Alliance for Regenerative Medicine (ARM). Innovation in the Time of COVID-19: H12020 ARM Global Regenerative Medicine and Advanced Therapy Sector Report. August 2020: https://alliancerm.org/sector-report/h1-2020-report-pdf Crossref
2. Statement from FDA Commissioner Scott Gottlieb, MD, and Peter Marks, MD, PhD, Director of the Center for Biologics Evaluation and Research, on new policies to advance development of safe and effective cell and gene therapies. January 2019: https://www.fda.gov/news-events/press-announcements/statement-fda-commissioner-scott-gottlieb-md-and-peter-marks-md-phd-director-center-biologics Crossref
3. Panch SR, Srivastava SK, Elavia N et al. Effect of cryopreservation on autologous chimeric antigen receptor T cell characteristics. Mol. Ther. 2019; 27(7): 1275. Crossref
4. Awan M, Buriak I, Fleck R et al. Dimethyl sulfoxide: a central player since the dawn of cryobiology, is efficacy balanced by toxicity? Regen. Med. 2020; 15(3): 1463. Crossref
5. ATCC website. FAQs: https://www.atcc.org/support/faqs/2820c/Osmolality+and+its+importance-54.aspx# Crossref
6. Baboo J, Kilbride P, Delahaye M et al. The impact of varying cooling and thawing rates on the quality of cryopreserved human peripheral blood T cells. Nature 2019; 9: 3417. Crossref
7. Yu G, Hubel A. The role of preservation in the variability of regenerative medicine products. Regen. Eng. Transl. Med. 2019; 5: 323. Crossref
8. Iyer RK, Bowles PA, Kim H, Dulgar-Tulloch A. Industrializing autologous adoptive immunotherapies: manufacturing advances and challenges. Front. Med. 2018; 5: 150. Crossref
9. Ball O, Robinson S, Bure K, Brindley DA, McCall D. Bioprocessing automation in cell therapy manufacturing: outcomes of special interest group automation workshop. Cytotherapy 2018; 20: 592. Crossref
10. Harrison RP, Medcalf N, Rafiq QA. Cell therapy-processing economics: small-scale micro factories as a stepping-stone toward large-scale macro factories. Regen. Med. 2018; 13(2): 159. Crossref
11. Levinson Y, Eylon Y, Heymann A, Zaretsky-Rits A, Karnieli O. Foundation elements for cell therapy smart scaling. BioProcess Int. 2015; 13(4)s. Crossref
12. Hornberger K, Yu G, McKenna D, Hubel A. Cryopreservation of hematopoietic stem cells: emerging assays, cryoprotectant agents, and technology to improve outcomes. Transfus. Med. Hemother. 2019; 46: 188. Crossref
13. Sethi D, Cunningham A, Massey K. Data-driven insights on cell therapy fill and finish processes. Genetic engineering & biotechnology news (webinar). July 23, 2020: https://www.genengnews.com/resources/webinars/data-driven-insights-on-cell-therapy-fill-and-finish-processes/ Crossref
Affiliations
Dalip Sethi
Manager, Scientific Affairs, Terumo Blood and Cell Technologies,
Inc, 10810 West Collins Avenue, Lakewood, CO 80215, USA
Annie Cunningham
Cell Therapy Laboratory Scientist, Terumo Blood and Cell Technologies, Inc, 10810 West Collins Avenue, Lakewood, CO 80215, USA
Authorship & Conflict of Interest
Contributions: All named authors take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Acknowledgements: None.
Disclosure and potential conflicts of interest: Dr Sethi and Ms Cunningham are both employees of Terumo Blood and Cell Technologies.
Funding declaration: The authors received no financial support for the research, authorship and/or publication of this article.
Article & copyright information
Copyright: Published by Cell and Gene Therapy Insights under Creative Commons License Deed CC BY NC ND 4.0 which allows anyone to copy, distribute, and transmit the article provided it is properly attributed in the manner specified below. No commercial use without permission.
Attribution: Copyright © 2020 Terumo Blood and Cell Technologies. Published by Cell and Gene Therapy Insights under Creative Commons License Deed CC BY NC ND 4.0.
Article source: Invited; externally peer reviewed.
Submitted for peer review: Oct 8 2020; Revised manuscript received: Nov 11 2020; Publication date: Nov 26 2020.

.png)