Filter by Interests
Filter by ContentType
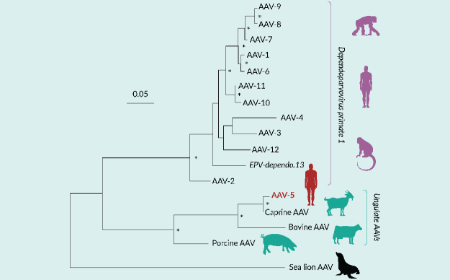
Unraveling the origins of human adeno-associated virus 5
Robert J Gifford, Robert M Kotin
14 June 2023
Commentary
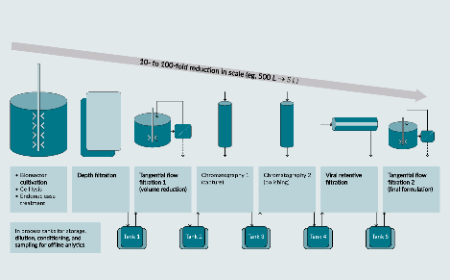
Opportunities to implement continuous processing in production of recombinant adeno-associated viral vectors
G Thakur, S Mink, H Bak et al
06 June 2023
Commentary
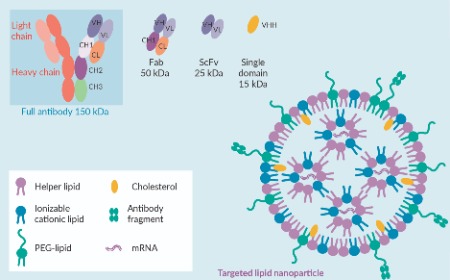
Therapeutic mRNA delivery with targeted lipid nanoparticles: next-generation transformative medicines
Umar Iqbal, Jagdeep K Sandhu
27 March 2022
Commentary
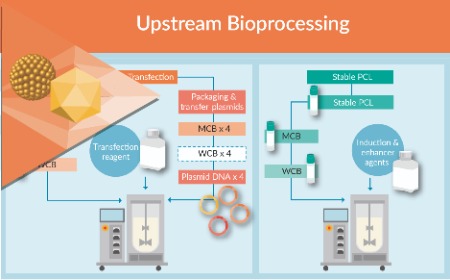
Enabling development of commercial-ready lentiviral vector manufacturing processes using stable producer cell lines
Peter Archibald, Ph.D, Anthony Shillings
22 September 2021
Commentary
Making the move from antibody therapeutics to gene therapy: applicability of monoclonal antibody learnings to adeno-associated virus vector bioprocessing
Andrew D Tustian
29 April 2021
Commentary

Considerations for performing virtual quality audits on manufacturers of gene therapy viral vectors: an auditor’s perspective during the COVID-19 public health emergency
Gary C du Moulin, PhD, MPH, RAC
18 March 2021
Commentary
Tools for tomorrow: expression without cells
Jade Tuck, Stuart Jamieson, Philip Probert
08 January 2021
Commentary
Two new capture options for improved purification of large mRNA
P Gagnon, B Goričar, Š Peršič et al
01 September 2020
Commentary

The right analytical toolbox is key to moving your pipeline forward
Andrew Espejo
25 August 2020
Commentary
Analytical Technology Used in the Latest Development of Gene Therapy Candidates
Z Li, Z Wu, T Maekawa et al
16 May 2019
Commentary