Biopreservation and cold chain biologistics risk points in the cell and gene therapy workflow
Cell & Gene Therapy Insights 2020; 6(08), 1363–1380
10.18609/cgti.2020.150
Introduction
The current state of regenerative medicine is a transformational period for cell and gene therapies. In addition to Novartis’ Kymriah®, Kite Pharma’s Yescarta® and Tecartus™, Spark’s Luxturna®, AveXis’ Zolgensma®, and bluebird bio’s Zynteglo® blazing the commercialization trail, there are over one thousand Phase 1, 2, and 3 cell and gene therapies (CGT) in pipeline development [1]Alliance for Regenerative Medicine Annual Report & Sector Year in Review: 2019.. Although this bodes well for patients, clinicians, industry, and investors, some unique aspects of cell- and gene-based therapies versus traditional pharmaceuticals or biopharma has highlighted the myriad of “new” manufacturing, clinical, and commercialization, challenges our industry now faces [2]Bersenev A, Kili S. Management of ‘out of specification’ commercial autologous CAR-T cell products. Cell Gene Ther. Ins. 2018; 4(11): 1051–8., [3]Chen LN, Collins-Johnson N, Sapp N, Pickett A, West K, Stroncek DF, Panch SR. How do I structure logistic processes in preparation for outsourcing of cellular therapy manufacturing? Transfusion 2019; 59: 2506–18. . Independently, each one of these challenges presents its own unique set of risks. Furthermore, when lined up in sequence and aggregated together in the manufacturing chain, if each portion is not optimized and risk-mitigated, the subsequent impact to the CGT product may be a compounding of the risks; and the sum total of all parts of the workflow will suffer. These beginning-to-end manufacturing risk points warrant appropriate assessment, and they are recommended to be addressed with the same diligence and priority as the therapies themselves, if the promise of Regenerative Medicine is to be fully realized. Fortunately, much has been learned regarding optimization of a number of key critical process parameters (CPP), and those looking to improve these parameters can leverage what has already been learned. This overview represents targeted lessons learned based on numerous experiences with CGT partners. Although intended to share feedback from experiences that may not always be detailed in the literature, it is not intended to address every aspect of the CGT workflow.
Representative cell immunotherapy workflow
Figure 1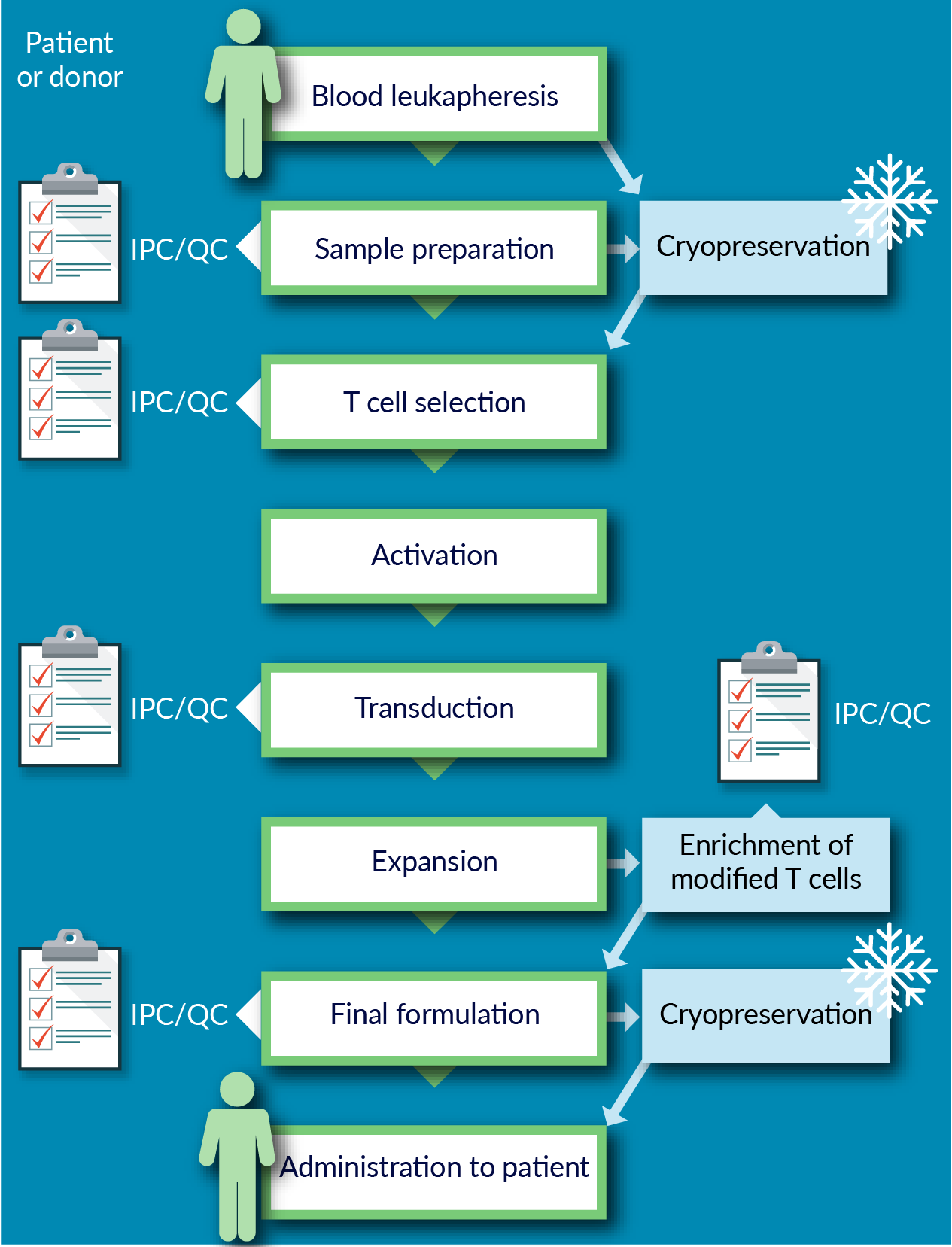
In common CGT manufacturing workflows, starting source material is obtained; and then is processed, selected, and/or isolated. Often, the material undergoes a biopreservation step (cryopreservation or hypothermic preservation), and transported to a manufacturing facility; where activation, transduction, expansion, and/or final formation take place, before additional transport/storage for clinical application. This workflow highlights several biopreservation and biologistics areas where CGT may be challenged:
- Ensuring high quality starting material;
- Optimizing viable functional recovery, and minimizing variability and risk, in process development throughout the workflow chain;
- Determining appropriate conditions for source material, intermediates, and final product – non-frozen or frozen (and, optimizing the biopreservation steps by utilizing Biopreservation Best Practices [5]Hawkins BJ, Abazari A, Mathew AJ. Biopreservation Best Practices for regenerative medicine GMP manufacturing & focus on optimized biopreservation media. Cell Gene Ther. Ins. 2017; 3(5): 345–58. Hawkins BJ, Abazari A, Mathew AJ. Biopreservation Best Practices for regenerative medicine GMP manufacturing & focus on optimized biopreservation media. Cell Gene Ther. Ins. 2017; 3(5): 345–58. Hawkins BJ, Abazari A, Mathew AJ. Biopreservation Best Practices for regenerative medicine GMP manufacturing & focus on optimized biopreservation media. Cell Gene Ther. Ins. 2017; 3(5): 345–58. Hawkins BJ, Abazari A, Mathew AJ. Biopreservation Best Practices for regenerative medicine GMP manufacturing & focus on optimized biopreservation media. Cell Gene Ther. Ins. 2017; 3(5): 345–58. Hawkins BJ, Abazari A, Mathew AJ. Biopreservation Best Practices for regenerative medicine GMP manufacturing & focus on optimized biopreservation media. Cell Gene Ther. Ins. 2017; 3(5): 345–58. Hawkins BJ, Abazari A, Mathew AJ. Biopreservation Best Practices for regenerative medicine GMP manufacturing & focus on optimized biopreservation media. Cell Gene Ther. Ins. 2017; 3(5): 345–58. Hawkins BJ, Abazari A, Mathew AJ. Biopreservation Best Practices for regenerative medicine GMP manufacturing & focus on optimized biopreservation media. Cell Gene Ther. Ins. 2017; 3(5): 345–58. Hawkins BJ, Abazari A, Mathew AJ. Biopreservation Best Practices for regenerative medicine GMP manufacturing & focus on optimized biopreservation media. Cell Gene Ther. Ins. 2017; 3(5): 345–58. Hawkins BJ, Abazari A, Mathew AJ. Biopreservation Best Practices for regenerative medicine GMP manufacturing & focus on optimized biopreservation media. Cell Gene Ther. Ins. 2017; 3(5): 345–58. Hawkins BJ, Abazari A, Mathew AJ. Biopreservation Best Practices for regenerative medicine GMP manufacturing & focus on optimized biopreservation media. Cell Gene Ther. Ins. 2017; 3(5): 345–58. Hawkins BJ, Abazari A, Mathew AJ. Biopreservation Best Practices for regenerative medicine GMP manufacturing & focus on optimized biopreservation media. Cell Gene Ther. Ins. 2017; 3(5): 345–58. Hawkins BJ, Abazari A, Mathew AJ. Biopreservation Best Practices for regenerative medicine GMP manufacturing & focus on optimized biopreservation media. Cell Gene Ther. Ins. 2017; 3(5): 345–58. Hawkins BJ, Abazari A, Mathew AJ. Biopreservation Best Practices for regenerative medicine GMP manufacturing & focus on optimized biopreservation media. Cell Gene Ther. Ins. 2017; 3(5): 345–58. Hawkins BJ, Abazari A, Mathew AJ. Biopreservation Best Practices for regenerative medicine GMP manufacturing & focus on optimized biopreservation media. Cell Gene Ther. Ins. 2017; 3(5): 345–58. Hawkins BJ, Abazari A, Mathew AJ. Biopreservation Best Practices for regenerative medicine GMP manufacturing & focus on optimized biopreservation media. Cell Gene Ther. Ins. 2017; 3(5): 345–58. Hawkins BJ, Abazari A, Mathew AJ. Biopreservation Best Practices for regenerative medicine GMP manufacturing & focus on optimized biopreservation media. Cell Gene Ther. Ins. 2017; 3(5): 345–58. ); and
- Exploring and implementing enabling tools and technologies throughout the workflow.
Such tools might consist of: novel CGT processing and packaging technologies; next generation closed systems for fill, finish, and packaging; class-defining biopreservation media; high capacity-controlled rate freezers; cryogenic storage systems; ‘smart’ cold chain management systems (shipping containers, tracking, and reporting); and automated, water-free thawing equipment technologies. [The normothermic culture state of the cells is also a variable that can impact the quality of the cell product, however that is not a focus of this overview.]
Ensuring high quality starting material
The importance of obtaining high quality starting material has been previously highlighted [4]Juliano L, Eastwood G, Berard T, Mathew AJ. The Importance of Collection, Processing and Biopreservation Best Practices in Determining CAR-T Starting Material Quality. Cell Gene Ther. Ins. 2018; 4(4): 327–36.Juliano L, Eastwood G, Berard T, Mathew AJ. The Importance of Collection, Processing and Biopreservation Best Practices in Determining CAR-T Starting Material Quality. Cell Gene Ther. Ins. 2018; 4(4): 327–36.Juliano L, Eastwood G, Berard T, Mathew AJ. The Importance of Collection, Processing and Biopreservation Best Practices in Determining CAR-T Starting Material Quality. Cell Gene Ther. Ins. 2018; 4(4): 327–36.Juliano L, Eastwood G, Berard T, Mathew AJ. The Importance of Collection, Processing and Biopreservation Best Practices in Determining CAR-T Starting Material Quality. Cell Gene Ther. Ins. 2018; 4(4): 327–36., [5]Hawkins BJ, Abazari A, Mathew AJ. Biopreservation Best Practices for regenerative medicine GMP manufacturing & focus on optimized biopreservation media. Cell Gene Ther. Ins. 2017; 3(5): 345–58. Hawkins BJ, Abazari A, Mathew AJ. Biopreservation Best Practices for regenerative medicine GMP manufacturing & focus on optimized biopreservation media. Cell Gene Ther. Ins. 2017; 3(5): 345–58. Hawkins BJ, Abazari A, Mathew AJ. Biopreservation Best Practices for regenerative medicine GMP manufacturing & focus on optimized biopreservation media. Cell Gene Ther. Ins. 2017; 3(5): 345–58. Hawkins BJ, Abazari A, Mathew AJ. Biopreservation Best Practices for regenerative medicine GMP manufacturing & focus on optimized biopreservation media. Cell Gene Ther. Ins. 2017; 3(5): 345–58. Hawkins BJ, Abazari A, Mathew AJ. Biopreservation Best Practices for regenerative medicine GMP manufacturing & focus on optimized biopreservation media. Cell Gene Ther. Ins. 2017; 3(5): 345–58. Hawkins BJ, Abazari A, Mathew AJ. Biopreservation Best Practices for regenerative medicine GMP manufacturing & focus on optimized biopreservation media. Cell Gene Ther. Ins. 2017; 3(5): 345–58. Hawkins BJ, Abazari A, Mathew AJ. Biopreservation Best Practices for regenerative medicine GMP manufacturing & focus on optimized biopreservation media. Cell Gene Ther. Ins. 2017; 3(5): 345–58. Hawkins BJ, Abazari A, Mathew AJ. Biopreservation Best Practices for regenerative medicine GMP manufacturing & focus on optimized biopreservation media. Cell Gene Ther. Ins. 2017; 3(5): 345–58. Hawkins BJ, Abazari A, Mathew AJ. Biopreservation Best Practices for regenerative medicine GMP manufacturing & focus on optimized biopreservation media. Cell Gene Ther. Ins. 2017; 3(5): 345–58. Hawkins BJ, Abazari A, Mathew AJ. Biopreservation Best Practices for regenerative medicine GMP manufacturing & focus on optimized biopreservation media. Cell Gene Ther. Ins. 2017; 3(5): 345–58. Hawkins BJ, Abazari A, Mathew AJ. Biopreservation Best Practices for regenerative medicine GMP manufacturing & focus on optimized biopreservation media. Cell Gene Ther. Ins. 2017; 3(5): 345–58. Hawkins BJ, Abazari A, Mathew AJ. Biopreservation Best Practices for regenerative medicine GMP manufacturing & focus on optimized biopreservation media. Cell Gene Ther. Ins. 2017; 3(5): 345–58. Hawkins BJ, Abazari A, Mathew AJ. Biopreservation Best Practices for regenerative medicine GMP manufacturing & focus on optimized biopreservation media. Cell Gene Ther. Ins. 2017; 3(5): 345–58. Hawkins BJ, Abazari A, Mathew AJ. Biopreservation Best Practices for regenerative medicine GMP manufacturing & focus on optimized biopreservation media. Cell Gene Ther. Ins. 2017; 3(5): 345–58. Hawkins BJ, Abazari A, Mathew AJ. Biopreservation Best Practices for regenerative medicine GMP manufacturing & focus on optimized biopreservation media. Cell Gene Ther. Ins. 2017; 3(5): 345–58. Hawkins BJ, Abazari A, Mathew AJ. Biopreservation Best Practices for regenerative medicine GMP manufacturing & focus on optimized biopreservation media. Cell Gene Ther. Ins. 2017; 3(5): 345–58. , [7]Clarke D, Smith D. Managing starting material stability to maximize manufacturing flexibility and downstream efficiency. Cell Gene Ther. Ins. 2019; 5(2): 303–14.Clarke D, Smith D. Managing starting material stability to maximize manufacturing flexibility and downstream efficiency. Cell Gene Ther. Ins. 2019; 5(2): 303–14.Clarke D, Smith D. Managing starting material stability to maximize manufacturing flexibility and downstream efficiency. Cell Gene Ther. Ins. 2019; 5(2): 303–14.Clarke D, Smith D. Managing starting material stability to maximize manufacturing flexibility and downstream efficiency. Cell Gene Ther. Ins. 2019; 5(2): 303–14.. An early challenge in the CGT manufacturing workflow is ensuring high quality, and consistent, starting material. Cell-based manufacturing and therapies present a unique challenge that does not exist to the same complexity or criticality as with non-cell-based therapies – that difference being the needs, the vulnerabilities, and variability of, living cells. Cells embody an intrinsic variability of normal conditions, response, and function, that can influence the therapeutic efficacy. As such, CGT manufacturing should take into account the inherent variability of starting cell-based materials, as well as the processing methods for these living cells, that will eventually impact the quality of the therapeutic product.
The potential variability and quality of CGT starting materials have been an increasing focus of CGT concern, and has been discussion points of Cell & Gene Therapy Insights experts [4]Juliano L, Eastwood G, Berard T, Mathew AJ. The Importance of Collection, Processing and Biopreservation Best Practices in Determining CAR-T Starting Material Quality. Cell Gene Ther. Ins. 2018; 4(4): 327–36.Juliano L, Eastwood G, Berard T, Mathew AJ. The Importance of Collection, Processing and Biopreservation Best Practices in Determining CAR-T Starting Material Quality. Cell Gene Ther. Ins. 2018; 4(4): 327–36.Juliano L, Eastwood G, Berard T, Mathew AJ. The Importance of Collection, Processing and Biopreservation Best Practices in Determining CAR-T Starting Material Quality. Cell Gene Ther. Ins. 2018; 4(4): 327–36.Juliano L, Eastwood G, Berard T, Mathew AJ. The Importance of Collection, Processing and Biopreservation Best Practices in Determining CAR-T Starting Material Quality. Cell Gene Ther. Ins. 2018; 4(4): 327–36., [7]Clarke D, Smith D. Managing starting material stability to maximize manufacturing flexibility and downstream efficiency. Cell Gene Ther. Ins. 2019; 5(2): 303–14.Clarke D, Smith D. Managing starting material stability to maximize manufacturing flexibility and downstream efficiency. Cell Gene Ther. Ins. 2019; 5(2): 303–14.Clarke D, Smith D. Managing starting material stability to maximize manufacturing flexibility and downstream efficiency. Cell Gene Ther. Ins. 2019; 5(2): 303–14.Clarke D, Smith D. Managing starting material stability to maximize manufacturing flexibility and downstream efficiency. Cell Gene Ther. Ins. 2019; 5(2): 303–14.. Those discussions have also presented evidence-based pathways for increasing the non-frozen or frozen stability, and/or minimizing variability, of cell/tissue starting materials [4]Juliano L, Eastwood G, Berard T, Mathew AJ. The Importance of Collection, Processing and Biopreservation Best Practices in Determining CAR-T Starting Material Quality. Cell Gene Ther. Ins. 2018; 4(4): 327–36.Juliano L, Eastwood G, Berard T, Mathew AJ. The Importance of Collection, Processing and Biopreservation Best Practices in Determining CAR-T Starting Material Quality. Cell Gene Ther. Ins. 2018; 4(4): 327–36.Juliano L, Eastwood G, Berard T, Mathew AJ. The Importance of Collection, Processing and Biopreservation Best Practices in Determining CAR-T Starting Material Quality. Cell Gene Ther. Ins. 2018; 4(4): 327–36.Juliano L, Eastwood G, Berard T, Mathew AJ. The Importance of Collection, Processing and Biopreservation Best Practices in Determining CAR-T Starting Material Quality. Cell Gene Ther. Ins. 2018; 4(4): 327–36., [5]Hawkins BJ, Abazari A, Mathew AJ. Biopreservation Best Practices for regenerative medicine GMP manufacturing & focus on optimized biopreservation media. Cell Gene Ther. Ins. 2017; 3(5): 345–58. Hawkins BJ, Abazari A, Mathew AJ. Biopreservation Best Practices for regenerative medicine GMP manufacturing & focus on optimized biopreservation media. Cell Gene Ther. Ins. 2017; 3(5): 345–58. Hawkins BJ, Abazari A, Mathew AJ. Biopreservation Best Practices for regenerative medicine GMP manufacturing & focus on optimized biopreservation media. Cell Gene Ther. Ins. 2017; 3(5): 345–58. Hawkins BJ, Abazari A, Mathew AJ. Biopreservation Best Practices for regenerative medicine GMP manufacturing & focus on optimized biopreservation media. Cell Gene Ther. Ins. 2017; 3(5): 345–58. Hawkins BJ, Abazari A, Mathew AJ. Biopreservation Best Practices for regenerative medicine GMP manufacturing & focus on optimized biopreservation media. Cell Gene Ther. Ins. 2017; 3(5): 345–58. Hawkins BJ, Abazari A, Mathew AJ. Biopreservation Best Practices for regenerative medicine GMP manufacturing & focus on optimized biopreservation media. Cell Gene Ther. Ins. 2017; 3(5): 345–58. Hawkins BJ, Abazari A, Mathew AJ. Biopreservation Best Practices for regenerative medicine GMP manufacturing & focus on optimized biopreservation media. Cell Gene Ther. Ins. 2017; 3(5): 345–58. Hawkins BJ, Abazari A, Mathew AJ. Biopreservation Best Practices for regenerative medicine GMP manufacturing & focus on optimized biopreservation media. Cell Gene Ther. Ins. 2017; 3(5): 345–58. Hawkins BJ, Abazari A, Mathew AJ. Biopreservation Best Practices for regenerative medicine GMP manufacturing & focus on optimized biopreservation media. Cell Gene Ther. Ins. 2017; 3(5): 345–58. Hawkins BJ, Abazari A, Mathew AJ. Biopreservation Best Practices for regenerative medicine GMP manufacturing & focus on optimized biopreservation media. Cell Gene Ther. Ins. 2017; 3(5): 345–58. Hawkins BJ, Abazari A, Mathew AJ. Biopreservation Best Practices for regenerative medicine GMP manufacturing & focus on optimized biopreservation media. Cell Gene Ther. Ins. 2017; 3(5): 345–58. Hawkins BJ, Abazari A, Mathew AJ. Biopreservation Best Practices for regenerative medicine GMP manufacturing & focus on optimized biopreservation media. Cell Gene Ther. Ins. 2017; 3(5): 345–58. Hawkins BJ, Abazari A, Mathew AJ. Biopreservation Best Practices for regenerative medicine GMP manufacturing & focus on optimized biopreservation media. Cell Gene Ther. Ins. 2017; 3(5): 345–58. Hawkins BJ, Abazari A, Mathew AJ. Biopreservation Best Practices for regenerative medicine GMP manufacturing & focus on optimized biopreservation media. Cell Gene Ther. Ins. 2017; 3(5): 345–58. Hawkins BJ, Abazari A, Mathew AJ. Biopreservation Best Practices for regenerative medicine GMP manufacturing & focus on optimized biopreservation media. Cell Gene Ther. Ins. 2017; 3(5): 345–58. Hawkins BJ, Abazari A, Mathew AJ. Biopreservation Best Practices for regenerative medicine GMP manufacturing & focus on optimized biopreservation media. Cell Gene Ther. Ins. 2017; 3(5): 345–58. , [6]Abazari A, Hawkins BJ, Clarke DM, Mathew AJ. Biopreservation Best Practices: A Cornerstone in the Supply Chain of Cell-based Therapies – MSC Model Case Study. Cell Gene Ther. Ins. 2017; 3(10): 853–71. Abazari A, Hawkins BJ, Clarke DM, Mathew AJ. Biopreservation Best Practices: A Cornerstone in the Supply Chain of Cell-based Therapies – MSC Model Case Study. Cell Gene Ther. Ins. 2017; 3(10): 853–71. Abazari A, Hawkins BJ, Clarke DM, Mathew AJ. Biopreservation Best Practices: A Cornerstone in the Supply Chain of Cell-based Therapies – MSC Model Case Study. Cell Gene Ther. Ins. 2017; 3(10): 853–71. , [7]Clarke D, Smith D. Managing starting material stability to maximize manufacturing flexibility and downstream efficiency. Cell Gene Ther. Ins. 2019; 5(2): 303–14.Clarke D, Smith D. Managing starting material stability to maximize manufacturing flexibility and downstream efficiency. Cell Gene Ther. Ins. 2019; 5(2): 303–14.Clarke D, Smith D. Managing starting material stability to maximize manufacturing flexibility and downstream efficiency. Cell Gene Ther. Ins. 2019; 5(2): 303–14.Clarke D, Smith D. Managing starting material stability to maximize manufacturing flexibility and downstream efficiency. Cell Gene Ther. Ins. 2019; 5(2): 303–14., [8]Abazari A. Process development considerations for cryopreservation of cellular therapies. Cell Gene Ther. Ins. 2019; 5(9): 1151–67.Abazari A. Process development considerations for cryopreservation of cellular therapies. Cell Gene Ther. Ins. 2019; 5(9): 1151–67.Abazari A. Process development considerations for cryopreservation of cellular therapies. Cell Gene Ther. Ins. 2019; 5(9): 1151–67.Abazari A. Process development considerations for cryopreservation of cellular therapies. Cell Gene Ther. Ins. 2019; 5(9): 1151–67.Abazari A. Process development considerations for cryopreservation of cellular therapies. Cell Gene Ther. Ins. 2019; 5(9): 1151–67.Abazari A. Process development considerations for cryopreservation of cellular therapies. Cell Gene Ther. Ins. 2019; 5(9): 1151–67.Abazari A. Process development considerations for cryopreservation of cellular therapies. Cell Gene Ther. Ins. 2019; 5(9): 1151–67.Abazari A. Process development considerations for cryopreservation of cellular therapies. Cell Gene Ther. Ins. 2019; 5(9): 1151–67.Abazari A. Process development considerations for cryopreservation of cellular therapies. Cell Gene Ther. Ins. 2019; 5(9): 1151–67., [9]Abazari A. Implementation of Biopreservation Best Practices to address a critical component of cell and gene therapy manufacturing: https://insights.bio/cell-and-gene-therapy-insights/implementation-of-biopreservation-best-practices-to-address-a-critical-component-of-cell-and-gene-therapy-manufacturing Abazari A. Implementation of Biopreservation Best Practices to address a critical component of cell and gene therapy manufacturing: https://insights.bio/cell-and-gene-therapy-insights/implementation-of-biopreservation-best-practices-to-address-a-critical-component-of-cell-and-gene-therapy-manufacturing Abazari A. Implementation of Biopreservation Best Practices to address a critical component of cell and gene therapy manufacturing: https://insights.bio/cell-and-gene-therapy-insights/implementation-of-biopreservation-best-practices-to-address-a-critical-component-of-cell-and-gene-therapy-manufacturing Abazari A. Implementation of Biopreservation Best Practices to address a critical component of cell and gene therapy manufacturing: https://insights.bio/cell-and-gene-therapy-insights/implementation-of-biopreservation-best-practices-to-address-a-critical-component-of-cell-and-gene-therapy-manufacturing Abazari A. Implementation of Biopreservation Best Practices to address a critical component of cell and gene therapy manufacturing: https://insights.bio/cell-and-gene-therapy-insights/implementation-of-biopreservation-best-practices-to-address-a-critical-component-of-cell-and-gene-therapy-manufacturing Abazari A. Implementation of Biopreservation Best Practices to address a critical component of cell and gene therapy manufacturing: https://insights.bio/cell-and-gene-therapy-insights/implementation-of-biopreservation-best-practices-to-address-a-critical-component-of-cell-and-gene-therapy-manufacturing Abazari A. Implementation of Biopreservation Best Practices to address a critical component of cell and gene therapy manufacturing: https://insights.bio/cell-and-gene-therapy-insights/implementation-of-biopreservation-best-practices-to-address-a-critical-component-of-cell-and-gene-therapy-manufacturing Abazari A. Implementation of Biopreservation Best Practices to address a critical component of cell and gene therapy manufacturing: https://insights.bio/cell-and-gene-therapy-insights/implementation-of-biopreservation-best-practices-to-address-a-critical-component-of-cell-and-gene-therapy-manufacturing Abazari A. Implementation of Biopreservation Best Practices to address a critical component of cell and gene therapy manufacturing: https://insights.bio/cell-and-gene-therapy-insights/implementation-of-biopreservation-best-practices-to-address-a-critical-component-of-cell-and-gene-therapy-manufacturing Abazari A. Implementation of Biopreservation Best Practices to address a critical component of cell and gene therapy manufacturing: https://insights.bio/cell-and-gene-therapy-insights/implementation-of-biopreservation-best-practices-to-address-a-critical-component-of-cell-and-gene-therapy-manufacturing Abazari A. Implementation of Biopreservation Best Practices to address a critical component of cell and gene therapy manufacturing: https://insights.bio/cell-and-gene-therapy-insights/implementation-of-biopreservation-best-practices-to-address-a-critical-component-of-cell-and-gene-therapy-manufacturing .
Non-frozen or frozen? Cellular responses to cold
It is important to ask a basic question: How can cell viable recovery and function be preserved throughout the manufacturing workflow, in order to facilitate efficacy? It is recognized that low temperatures can slow metabolic activity, reduce oxygen demand, and decrease degradation; but it may be beneficial to understand the benefits and limitations, in order to support biopreservation optimization and risk management of the process/product.
Figure 2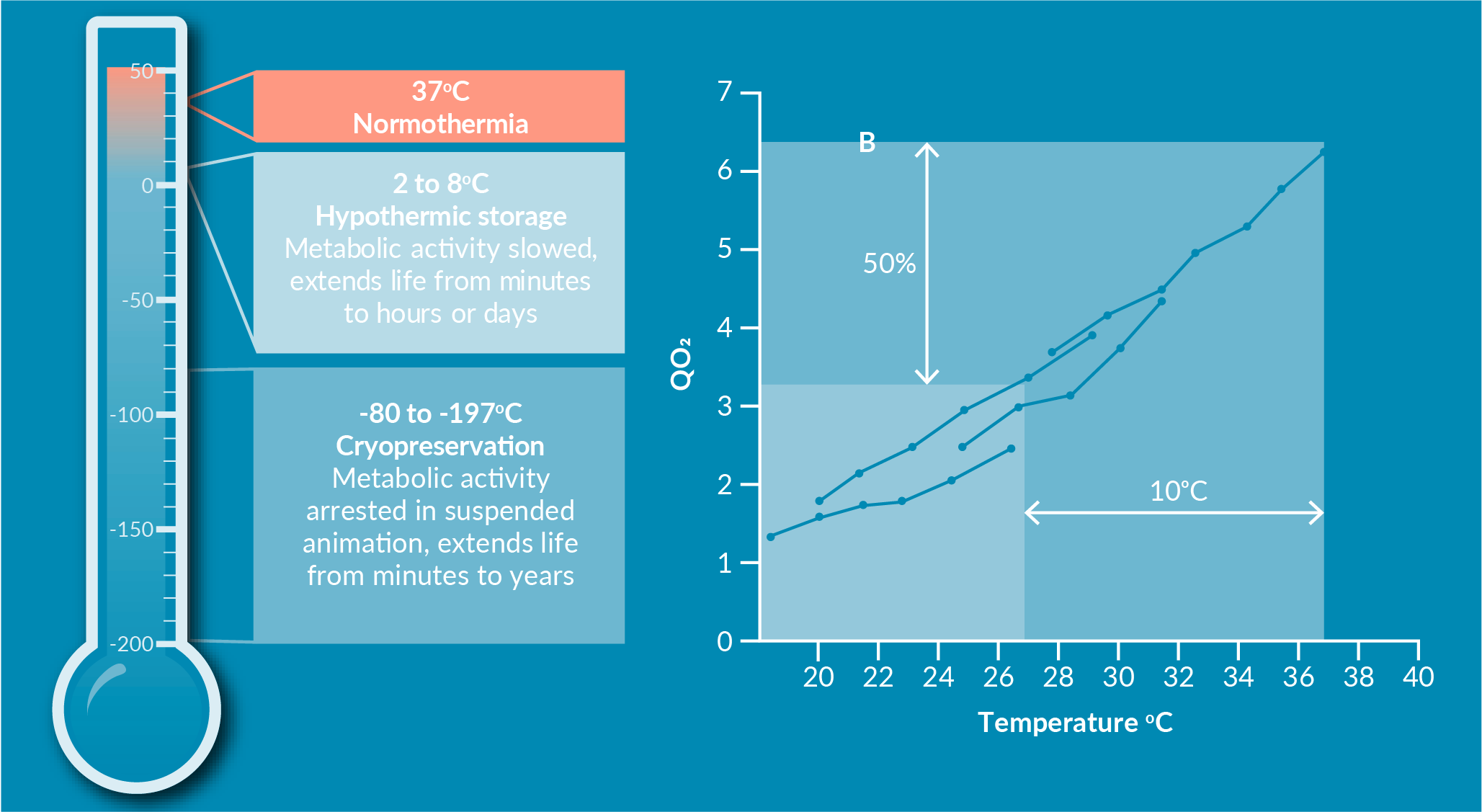
As temperatures decrease to hypothermic temperatures (below 37°C normothermic), lipid membranes undergo phase transitions: a type of structural change that results in loss of fluidity and continuity. Hypothermia induces phase transitions in the lipid membrane that lead to pore formation and loss of integrity. This leads to an influx and outflux of ions and small molecules due to the cross-membrane concentration gradients [9]Abazari A. Implementation of Biopreservation Best Practices to address a critical component of cell and gene therapy manufacturing: https://insights.bio/cell-and-gene-therapy-insights/implementation-of-biopreservation-best-practices-to-address-a-critical-component-of-cell-and-gene-therapy-manufacturing Abazari A. Implementation of Biopreservation Best Practices to address a critical component of cell and gene therapy manufacturing: https://insights.bio/cell-and-gene-therapy-insights/implementation-of-biopreservation-best-practices-to-address-a-critical-component-of-cell-and-gene-therapy-manufacturing Abazari A. Implementation of Biopreservation Best Practices to address a critical component of cell and gene therapy manufacturing: https://insights.bio/cell-and-gene-therapy-insights/implementation-of-biopreservation-best-practices-to-address-a-critical-component-of-cell-and-gene-therapy-manufacturing Abazari A. Implementation of Biopreservation Best Practices to address a critical component of cell and gene therapy manufacturing: https://insights.bio/cell-and-gene-therapy-insights/implementation-of-biopreservation-best-practices-to-address-a-critical-component-of-cell-and-gene-therapy-manufacturing Abazari A. Implementation of Biopreservation Best Practices to address a critical component of cell and gene therapy manufacturing: https://insights.bio/cell-and-gene-therapy-insights/implementation-of-biopreservation-best-practices-to-address-a-critical-component-of-cell-and-gene-therapy-manufacturing Abazari A. Implementation of Biopreservation Best Practices to address a critical component of cell and gene therapy manufacturing: https://insights.bio/cell-and-gene-therapy-insights/implementation-of-biopreservation-best-practices-to-address-a-critical-component-of-cell-and-gene-therapy-manufacturing Abazari A. Implementation of Biopreservation Best Practices to address a critical component of cell and gene therapy manufacturing: https://insights.bio/cell-and-gene-therapy-insights/implementation-of-biopreservation-best-practices-to-address-a-critical-component-of-cell-and-gene-therapy-manufacturing Abazari A. Implementation of Biopreservation Best Practices to address a critical component of cell and gene therapy manufacturing: https://insights.bio/cell-and-gene-therapy-insights/implementation-of-biopreservation-best-practices-to-address-a-critical-component-of-cell-and-gene-therapy-manufacturing Abazari A. Implementation of Biopreservation Best Practices to address a critical component of cell and gene therapy manufacturing: https://insights.bio/cell-and-gene-therapy-insights/implementation-of-biopreservation-best-practices-to-address-a-critical-component-of-cell-and-gene-therapy-manufacturing Abazari A. Implementation of Biopreservation Best Practices to address a critical component of cell and gene therapy manufacturing: https://insights.bio/cell-and-gene-therapy-insights/implementation-of-biopreservation-best-practices-to-address-a-critical-component-of-cell-and-gene-therapy-manufacturing Abazari A. Implementation of Biopreservation Best Practices to address a critical component of cell and gene therapy manufacturing: https://insights.bio/cell-and-gene-therapy-insights/implementation-of-biopreservation-best-practices-to-address-a-critical-component-of-cell-and-gene-therapy-manufacturing . Under hypothermic conditions, there is deceleration of ion pumps and reduced ATP synthesis by mitochondria. Ion pumps then have a reduced capacity to regulate intracellular ions, leading to a myriad of issues. This further impedes restoration of ionic balance in the intracellular milieu. This disrupts the overall ionic balance, resulting in dysfunctions in intracellular cell signaling, salinity, osmolality pathways, osmosis, and cell volume, that previously relied on a tightly regulated cell balance. Osmolality and ionic distortions can induce mitochondrial stresses, which can initiate a cascade of adverse events within the cell by increased reactive oxygen species (ROS) and free radicals generation, and lipid peroxidation. When combined with membrane phase transitions, these phenomena can lead to membrane blebbing and other irreversible membrane injuries, among other mechanisms of cell damage and cell death [5]Hawkins BJ, Abazari A, Mathew AJ. Biopreservation Best Practices for regenerative medicine GMP manufacturing & focus on optimized biopreservation media. Cell Gene Ther. Ins. 2017; 3(5): 345–58. Hawkins BJ, Abazari A, Mathew AJ. Biopreservation Best Practices for regenerative medicine GMP manufacturing & focus on optimized biopreservation media. Cell Gene Ther. Ins. 2017; 3(5): 345–58. Hawkins BJ, Abazari A, Mathew AJ. Biopreservation Best Practices for regenerative medicine GMP manufacturing & focus on optimized biopreservation media. Cell Gene Ther. Ins. 2017; 3(5): 345–58. Hawkins BJ, Abazari A, Mathew AJ. Biopreservation Best Practices for regenerative medicine GMP manufacturing & focus on optimized biopreservation media. Cell Gene Ther. Ins. 2017; 3(5): 345–58. Hawkins BJ, Abazari A, Mathew AJ. Biopreservation Best Practices for regenerative medicine GMP manufacturing & focus on optimized biopreservation media. Cell Gene Ther. Ins. 2017; 3(5): 345–58. Hawkins BJ, Abazari A, Mathew AJ. Biopreservation Best Practices for regenerative medicine GMP manufacturing & focus on optimized biopreservation media. Cell Gene Ther. Ins. 2017; 3(5): 345–58. Hawkins BJ, Abazari A, Mathew AJ. Biopreservation Best Practices for regenerative medicine GMP manufacturing & focus on optimized biopreservation media. Cell Gene Ther. Ins. 2017; 3(5): 345–58. Hawkins BJ, Abazari A, Mathew AJ. Biopreservation Best Practices for regenerative medicine GMP manufacturing & focus on optimized biopreservation media. Cell Gene Ther. Ins. 2017; 3(5): 345–58. Hawkins BJ, Abazari A, Mathew AJ. Biopreservation Best Practices for regenerative medicine GMP manufacturing & focus on optimized biopreservation media. Cell Gene Ther. Ins. 2017; 3(5): 345–58. Hawkins BJ, Abazari A, Mathew AJ. Biopreservation Best Practices for regenerative medicine GMP manufacturing & focus on optimized biopreservation media. Cell Gene Ther. Ins. 2017; 3(5): 345–58. Hawkins BJ, Abazari A, Mathew AJ. Biopreservation Best Practices for regenerative medicine GMP manufacturing & focus on optimized biopreservation media. Cell Gene Ther. Ins. 2017; 3(5): 345–58. Hawkins BJ, Abazari A, Mathew AJ. Biopreservation Best Practices for regenerative medicine GMP manufacturing & focus on optimized biopreservation media. Cell Gene Ther. Ins. 2017; 3(5): 345–58. Hawkins BJ, Abazari A, Mathew AJ. Biopreservation Best Practices for regenerative medicine GMP manufacturing & focus on optimized biopreservation media. Cell Gene Ther. Ins. 2017; 3(5): 345–58. Hawkins BJ, Abazari A, Mathew AJ. Biopreservation Best Practices for regenerative medicine GMP manufacturing & focus on optimized biopreservation media. Cell Gene Ther. Ins. 2017; 3(5): 345–58. Hawkins BJ, Abazari A, Mathew AJ. Biopreservation Best Practices for regenerative medicine GMP manufacturing & focus on optimized biopreservation media. Cell Gene Ther. Ins. 2017; 3(5): 345–58. Hawkins BJ, Abazari A, Mathew AJ. Biopreservation Best Practices for regenerative medicine GMP manufacturing & focus on optimized biopreservation media. Cell Gene Ther. Ins. 2017; 3(5): 345–58. , [9]Abazari A. Implementation of Biopreservation Best Practices to address a critical component of cell and gene therapy manufacturing: https://insights.bio/cell-and-gene-therapy-insights/implementation-of-biopreservation-best-practices-to-address-a-critical-component-of-cell-and-gene-therapy-manufacturing Abazari A. Implementation of Biopreservation Best Practices to address a critical component of cell and gene therapy manufacturing: https://insights.bio/cell-and-gene-therapy-insights/implementation-of-biopreservation-best-practices-to-address-a-critical-component-of-cell-and-gene-therapy-manufacturing Abazari A. Implementation of Biopreservation Best Practices to address a critical component of cell and gene therapy manufacturing: https://insights.bio/cell-and-gene-therapy-insights/implementation-of-biopreservation-best-practices-to-address-a-critical-component-of-cell-and-gene-therapy-manufacturing Abazari A. Implementation of Biopreservation Best Practices to address a critical component of cell and gene therapy manufacturing: https://insights.bio/cell-and-gene-therapy-insights/implementation-of-biopreservation-best-practices-to-address-a-critical-component-of-cell-and-gene-therapy-manufacturing Abazari A. Implementation of Biopreservation Best Practices to address a critical component of cell and gene therapy manufacturing: https://insights.bio/cell-and-gene-therapy-insights/implementation-of-biopreservation-best-practices-to-address-a-critical-component-of-cell-and-gene-therapy-manufacturing Abazari A. Implementation of Biopreservation Best Practices to address a critical component of cell and gene therapy manufacturing: https://insights.bio/cell-and-gene-therapy-insights/implementation-of-biopreservation-best-practices-to-address-a-critical-component-of-cell-and-gene-therapy-manufacturing Abazari A. Implementation of Biopreservation Best Practices to address a critical component of cell and gene therapy manufacturing: https://insights.bio/cell-and-gene-therapy-insights/implementation-of-biopreservation-best-practices-to-address-a-critical-component-of-cell-and-gene-therapy-manufacturing Abazari A. Implementation of Biopreservation Best Practices to address a critical component of cell and gene therapy manufacturing: https://insights.bio/cell-and-gene-therapy-insights/implementation-of-biopreservation-best-practices-to-address-a-critical-component-of-cell-and-gene-therapy-manufacturing Abazari A. Implementation of Biopreservation Best Practices to address a critical component of cell and gene therapy manufacturing: https://insights.bio/cell-and-gene-therapy-insights/implementation-of-biopreservation-best-practices-to-address-a-critical-component-of-cell-and-gene-therapy-manufacturing Abazari A. Implementation of Biopreservation Best Practices to address a critical component of cell and gene therapy manufacturing: https://insights.bio/cell-and-gene-therapy-insights/implementation-of-biopreservation-best-practices-to-address-a-critical-component-of-cell-and-gene-therapy-manufacturing Abazari A. Implementation of Biopreservation Best Practices to address a critical component of cell and gene therapy manufacturing: https://insights.bio/cell-and-gene-therapy-insights/implementation-of-biopreservation-best-practices-to-address-a-critical-component-of-cell-and-gene-therapy-manufacturing , [10]Mathew AJ. Biopreservation Considerations for Regenerative Medicine GMP Manufacturing; 2018: http://ebook.liebertpub.com/biolife-solutions/biopreservation-considerations-for-regenerative-medicine-gmp-manufacturing Mathew AJ. Biopreservation Considerations for Regenerative Medicine GMP Manufacturing; 2018: http://ebook.liebertpub.com/biolife-solutions/biopreservation-considerations-for-regenerative-medicine-gmp-manufacturing Mathew AJ. Biopreservation Considerations for Regenerative Medicine GMP Manufacturing; 2018: http://ebook.liebertpub.com/biolife-solutions/biopreservation-considerations-for-regenerative-medicine-gmp-manufacturing Mathew AJ. Biopreservation Considerations for Regenerative Medicine GMP Manufacturing; 2018: http://ebook.liebertpub.com/biolife-solutions/biopreservation-considerations-for-regenerative-medicine-gmp-manufacturing Mathew AJ. Biopreservation Considerations for Regenerative Medicine GMP Manufacturing; 2018: http://ebook.liebertpub.com/biolife-solutions/biopreservation-considerations-for-regenerative-medicine-gmp-manufacturing .
Furthermore, in the absence of oxygen and normothermic conditions, glycolysis becomes the main source of limited ATP generation instead of oxidative phosphorylation, resulting in acidification of the intracellular milieu. Changes in pH and salinity may irreversibly impact protein solubility and its functional structures, which are necessary for protein-protein interactions and trans-membrane positioning.
Temporal accumulation of these damages during hypothermic intervals and storage may eventually overflow beyond the tolerable limits for the cell, leading to irreversible activation of apoptosis, necrosis, and secondary necrosis cascades; at which point, the cell is lost. In addition, the actual onset of cell damage and cell death may not translate until post-preservation and re-warming, and may subsequently manifest as Delayed Onset Cell Death [5]Hawkins BJ, Abazari A, Mathew AJ. Biopreservation Best Practices for regenerative medicine GMP manufacturing & focus on optimized biopreservation media. Cell Gene Ther. Ins. 2017; 3(5): 345–58. Hawkins BJ, Abazari A, Mathew AJ. Biopreservation Best Practices for regenerative medicine GMP manufacturing & focus on optimized biopreservation media. Cell Gene Ther. Ins. 2017; 3(5): 345–58. Hawkins BJ, Abazari A, Mathew AJ. Biopreservation Best Practices for regenerative medicine GMP manufacturing & focus on optimized biopreservation media. Cell Gene Ther. Ins. 2017; 3(5): 345–58. Hawkins BJ, Abazari A, Mathew AJ. Biopreservation Best Practices for regenerative medicine GMP manufacturing & focus on optimized biopreservation media. Cell Gene Ther. Ins. 2017; 3(5): 345–58. Hawkins BJ, Abazari A, Mathew AJ. Biopreservation Best Practices for regenerative medicine GMP manufacturing & focus on optimized biopreservation media. Cell Gene Ther. Ins. 2017; 3(5): 345–58. Hawkins BJ, Abazari A, Mathew AJ. Biopreservation Best Practices for regenerative medicine GMP manufacturing & focus on optimized biopreservation media. Cell Gene Ther. Ins. 2017; 3(5): 345–58. Hawkins BJ, Abazari A, Mathew AJ. Biopreservation Best Practices for regenerative medicine GMP manufacturing & focus on optimized biopreservation media. Cell Gene Ther. Ins. 2017; 3(5): 345–58. Hawkins BJ, Abazari A, Mathew AJ. Biopreservation Best Practices for regenerative medicine GMP manufacturing & focus on optimized biopreservation media. Cell Gene Ther. Ins. 2017; 3(5): 345–58. Hawkins BJ, Abazari A, Mathew AJ. Biopreservation Best Practices for regenerative medicine GMP manufacturing & focus on optimized biopreservation media. Cell Gene Ther. Ins. 2017; 3(5): 345–58. Hawkins BJ, Abazari A, Mathew AJ. Biopreservation Best Practices for regenerative medicine GMP manufacturing & focus on optimized biopreservation media. Cell Gene Ther. Ins. 2017; 3(5): 345–58. Hawkins BJ, Abazari A, Mathew AJ. Biopreservation Best Practices for regenerative medicine GMP manufacturing & focus on optimized biopreservation media. Cell Gene Ther. Ins. 2017; 3(5): 345–58. Hawkins BJ, Abazari A, Mathew AJ. Biopreservation Best Practices for regenerative medicine GMP manufacturing & focus on optimized biopreservation media. Cell Gene Ther. Ins. 2017; 3(5): 345–58. Hawkins BJ, Abazari A, Mathew AJ. Biopreservation Best Practices for regenerative medicine GMP manufacturing & focus on optimized biopreservation media. Cell Gene Ther. Ins. 2017; 3(5): 345–58. Hawkins BJ, Abazari A, Mathew AJ. Biopreservation Best Practices for regenerative medicine GMP manufacturing & focus on optimized biopreservation media. Cell Gene Ther. Ins. 2017; 3(5): 345–58. Hawkins BJ, Abazari A, Mathew AJ. Biopreservation Best Practices for regenerative medicine GMP manufacturing & focus on optimized biopreservation media. Cell Gene Ther. Ins. 2017; 3(5): 345–58. Hawkins BJ, Abazari A, Mathew AJ. Biopreservation Best Practices for regenerative medicine GMP manufacturing & focus on optimized biopreservation media. Cell Gene Ther. Ins. 2017; 3(5): 345–58. , [10]Mathew AJ. Biopreservation Considerations for Regenerative Medicine GMP Manufacturing; 2018: http://ebook.liebertpub.com/biolife-solutions/biopreservation-considerations-for-regenerative-medicine-gmp-manufacturing Mathew AJ. Biopreservation Considerations for Regenerative Medicine GMP Manufacturing; 2018: http://ebook.liebertpub.com/biolife-solutions/biopreservation-considerations-for-regenerative-medicine-gmp-manufacturing Mathew AJ. Biopreservation Considerations for Regenerative Medicine GMP Manufacturing; 2018: http://ebook.liebertpub.com/biolife-solutions/biopreservation-considerations-for-regenerative-medicine-gmp-manufacturing Mathew AJ. Biopreservation Considerations for Regenerative Medicine GMP Manufacturing; 2018: http://ebook.liebertpub.com/biolife-solutions/biopreservation-considerations-for-regenerative-medicine-gmp-manufacturing Mathew AJ. Biopreservation Considerations for Regenerative Medicine GMP Manufacturing; 2018: http://ebook.liebertpub.com/biolife-solutions/biopreservation-considerations-for-regenerative-medicine-gmp-manufacturing .
To alleviate some of these issues, an intracellular-like designed biopreservation media may be incorporated to replace traditional saline/culture media (or other formulations that mimic the normothermic isotonic ionic balance). By reducing the cross-membrane concentration gradient of ions during cold exposure, intracellular ionic balance and salinity would be less altered, even if membrane permeability is impacted. Biopreservation Critical Quality Attributes (BCQA) incorporate intracellular-like design, including impermeant (non-permeating) molecules such as large sugars, which exert membrane-stabilizing and osmotic-supporting effects, in order to mitigate cell swelling and membrane damage during storage. Free radical scavengers can decrease the burden of ROS. Also, buffers that are effective specifically at low temperatures, in contrast to traditional buffers for normothermic conditions, may be more effective at controlling toxic pH changes [5]Hawkins BJ, Abazari A, Mathew AJ. Biopreservation Best Practices for regenerative medicine GMP manufacturing & focus on optimized biopreservation media. Cell Gene Ther. Ins. 2017; 3(5): 345–58. Hawkins BJ, Abazari A, Mathew AJ. Biopreservation Best Practices for regenerative medicine GMP manufacturing & focus on optimized biopreservation media. Cell Gene Ther. Ins. 2017; 3(5): 345–58. Hawkins BJ, Abazari A, Mathew AJ. Biopreservation Best Practices for regenerative medicine GMP manufacturing & focus on optimized biopreservation media. Cell Gene Ther. Ins. 2017; 3(5): 345–58. Hawkins BJ, Abazari A, Mathew AJ. Biopreservation Best Practices for regenerative medicine GMP manufacturing & focus on optimized biopreservation media. Cell Gene Ther. Ins. 2017; 3(5): 345–58. Hawkins BJ, Abazari A, Mathew AJ. Biopreservation Best Practices for regenerative medicine GMP manufacturing & focus on optimized biopreservation media. Cell Gene Ther. Ins. 2017; 3(5): 345–58. Hawkins BJ, Abazari A, Mathew AJ. Biopreservation Best Practices for regenerative medicine GMP manufacturing & focus on optimized biopreservation media. Cell Gene Ther. Ins. 2017; 3(5): 345–58. Hawkins BJ, Abazari A, Mathew AJ. Biopreservation Best Practices for regenerative medicine GMP manufacturing & focus on optimized biopreservation media. Cell Gene Ther. Ins. 2017; 3(5): 345–58. Hawkins BJ, Abazari A, Mathew AJ. Biopreservation Best Practices for regenerative medicine GMP manufacturing & focus on optimized biopreservation media. Cell Gene Ther. Ins. 2017; 3(5): 345–58. Hawkins BJ, Abazari A, Mathew AJ. Biopreservation Best Practices for regenerative medicine GMP manufacturing & focus on optimized biopreservation media. Cell Gene Ther. Ins. 2017; 3(5): 345–58. Hawkins BJ, Abazari A, Mathew AJ. Biopreservation Best Practices for regenerative medicine GMP manufacturing & focus on optimized biopreservation media. Cell Gene Ther. Ins. 2017; 3(5): 345–58. Hawkins BJ, Abazari A, Mathew AJ. Biopreservation Best Practices for regenerative medicine GMP manufacturing & focus on optimized biopreservation media. Cell Gene Ther. Ins. 2017; 3(5): 345–58. Hawkins BJ, Abazari A, Mathew AJ. Biopreservation Best Practices for regenerative medicine GMP manufacturing & focus on optimized biopreservation media. Cell Gene Ther. Ins. 2017; 3(5): 345–58. Hawkins BJ, Abazari A, Mathew AJ. Biopreservation Best Practices for regenerative medicine GMP manufacturing & focus on optimized biopreservation media. Cell Gene Ther. Ins. 2017; 3(5): 345–58. Hawkins BJ, Abazari A, Mathew AJ. Biopreservation Best Practices for regenerative medicine GMP manufacturing & focus on optimized biopreservation media. Cell Gene Ther. Ins. 2017; 3(5): 345–58. Hawkins BJ, Abazari A, Mathew AJ. Biopreservation Best Practices for regenerative medicine GMP manufacturing & focus on optimized biopreservation media. Cell Gene Ther. Ins. 2017; 3(5): 345–58. Hawkins BJ, Abazari A, Mathew AJ. Biopreservation Best Practices for regenerative medicine GMP manufacturing & focus on optimized biopreservation media. Cell Gene Ther. Ins. 2017; 3(5): 345–58. . This intracellular-like approach to Biopreservation Best Practices is applicable to non-frozen hypothermic preservation and cryopreservation.
The physics of freezing
Another mode of cell and tissue biopreservation is cryopreservation. Hypothermia-induced acute stresses occur slowly and accumulate during the storage period. The accumulation of such adverse effects on cells usually trigger cell damage and cell death after hours to days in cold storage. On the other hand, acute cellular stresses during freezing conditions and cryopreservation occur within a relatively short period of freeze-thaw. For both modes of biopreservation, many cell damage and cell death effectors may only fully manifest over 24–72 hours post-preservation via Delayed Onset Cell Death [5]Hawkins BJ, Abazari A, Mathew AJ. Biopreservation Best Practices for regenerative medicine GMP manufacturing & focus on optimized biopreservation media. Cell Gene Ther. Ins. 2017; 3(5): 345–58. Hawkins BJ, Abazari A, Mathew AJ. Biopreservation Best Practices for regenerative medicine GMP manufacturing & focus on optimized biopreservation media. Cell Gene Ther. Ins. 2017; 3(5): 345–58. Hawkins BJ, Abazari A, Mathew AJ. Biopreservation Best Practices for regenerative medicine GMP manufacturing & focus on optimized biopreservation media. Cell Gene Ther. Ins. 2017; 3(5): 345–58. Hawkins BJ, Abazari A, Mathew AJ. Biopreservation Best Practices for regenerative medicine GMP manufacturing & focus on optimized biopreservation media. Cell Gene Ther. Ins. 2017; 3(5): 345–58. Hawkins BJ, Abazari A, Mathew AJ. Biopreservation Best Practices for regenerative medicine GMP manufacturing & focus on optimized biopreservation media. Cell Gene Ther. Ins. 2017; 3(5): 345–58. Hawkins BJ, Abazari A, Mathew AJ. Biopreservation Best Practices for regenerative medicine GMP manufacturing & focus on optimized biopreservation media. Cell Gene Ther. Ins. 2017; 3(5): 345–58. Hawkins BJ, Abazari A, Mathew AJ. Biopreservation Best Practices for regenerative medicine GMP manufacturing & focus on optimized biopreservation media. Cell Gene Ther. Ins. 2017; 3(5): 345–58. Hawkins BJ, Abazari A, Mathew AJ. Biopreservation Best Practices for regenerative medicine GMP manufacturing & focus on optimized biopreservation media. Cell Gene Ther. Ins. 2017; 3(5): 345–58. Hawkins BJ, Abazari A, Mathew AJ. Biopreservation Best Practices for regenerative medicine GMP manufacturing & focus on optimized biopreservation media. Cell Gene Ther. Ins. 2017; 3(5): 345–58. Hawkins BJ, Abazari A, Mathew AJ. Biopreservation Best Practices for regenerative medicine GMP manufacturing & focus on optimized biopreservation media. Cell Gene Ther. Ins. 2017; 3(5): 345–58. Hawkins BJ, Abazari A, Mathew AJ. Biopreservation Best Practices for regenerative medicine GMP manufacturing & focus on optimized biopreservation media. Cell Gene Ther. Ins. 2017; 3(5): 345–58. Hawkins BJ, Abazari A, Mathew AJ. Biopreservation Best Practices for regenerative medicine GMP manufacturing & focus on optimized biopreservation media. Cell Gene Ther. Ins. 2017; 3(5): 345–58. Hawkins BJ, Abazari A, Mathew AJ. Biopreservation Best Practices for regenerative medicine GMP manufacturing & focus on optimized biopreservation media. Cell Gene Ther. Ins. 2017; 3(5): 345–58. Hawkins BJ, Abazari A, Mathew AJ. Biopreservation Best Practices for regenerative medicine GMP manufacturing & focus on optimized biopreservation media. Cell Gene Ther. Ins. 2017; 3(5): 345–58. Hawkins BJ, Abazari A, Mathew AJ. Biopreservation Best Practices for regenerative medicine GMP manufacturing & focus on optimized biopreservation media. Cell Gene Ther. Ins. 2017; 3(5): 345–58. Hawkins BJ, Abazari A, Mathew AJ. Biopreservation Best Practices for regenerative medicine GMP manufacturing & focus on optimized biopreservation media. Cell Gene Ther. Ins. 2017; 3(5): 345–58. , [10]Mathew AJ. Biopreservation Considerations for Regenerative Medicine GMP Manufacturing; 2018: http://ebook.liebertpub.com/biolife-solutions/biopreservation-considerations-for-regenerative-medicine-gmp-manufacturing Mathew AJ. Biopreservation Considerations for Regenerative Medicine GMP Manufacturing; 2018: http://ebook.liebertpub.com/biolife-solutions/biopreservation-considerations-for-regenerative-medicine-gmp-manufacturing Mathew AJ. Biopreservation Considerations for Regenerative Medicine GMP Manufacturing; 2018: http://ebook.liebertpub.com/biolife-solutions/biopreservation-considerations-for-regenerative-medicine-gmp-manufacturing Mathew AJ. Biopreservation Considerations for Regenerative Medicine GMP Manufacturing; 2018: http://ebook.liebertpub.com/biolife-solutions/biopreservation-considerations-for-regenerative-medicine-gmp-manufacturing Mathew AJ. Biopreservation Considerations for Regenerative Medicine GMP Manufacturing; 2018: http://ebook.liebertpub.com/biolife-solutions/biopreservation-considerations-for-regenerative-medicine-gmp-manufacturing . To better understand the physical and chemical stresses during freezing conditions, consider a cell suspension in a simple salt solution such as physiological saline. In Figure 3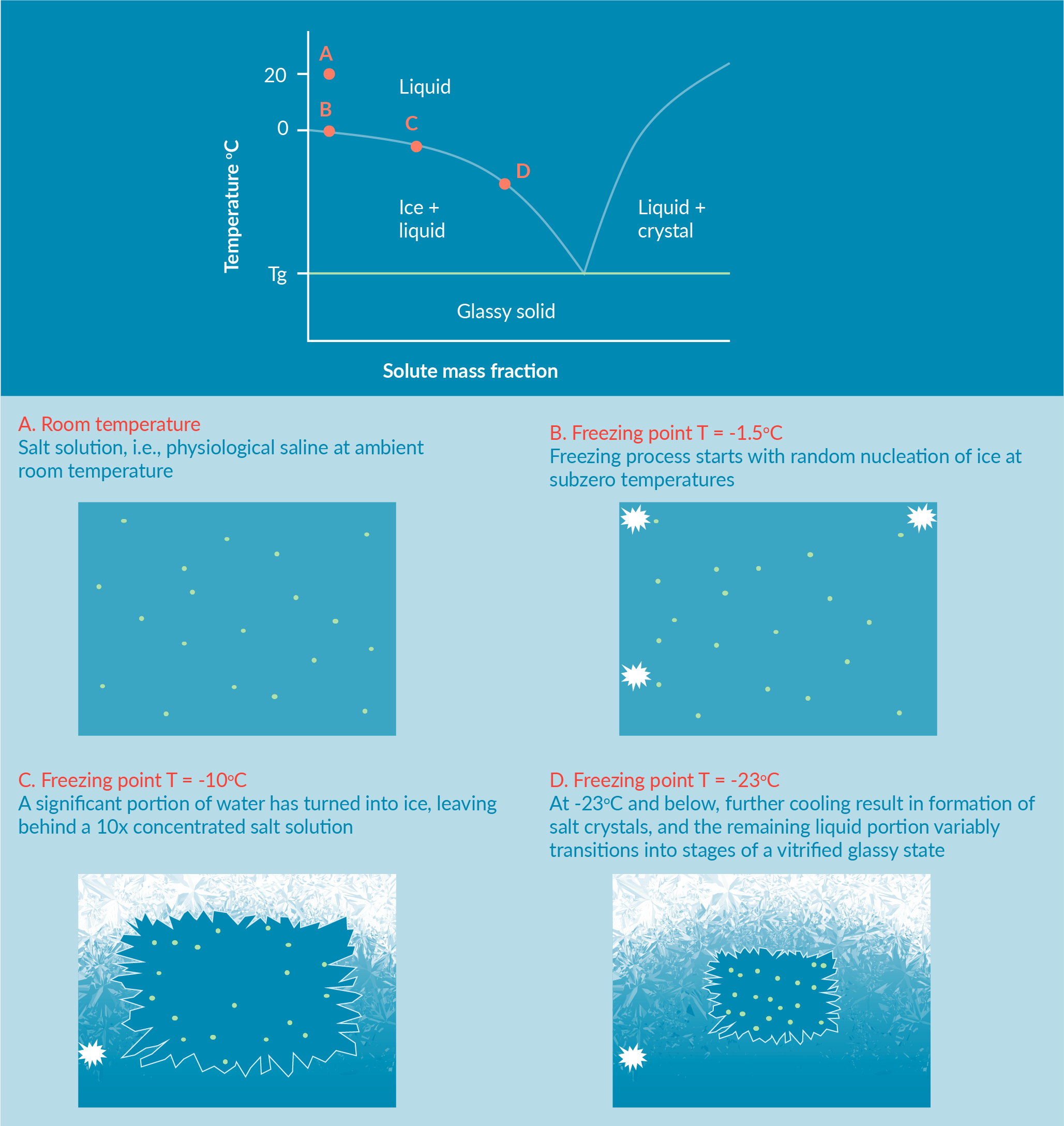
The freezing process starts with cooling the solution to below its freezing point (Figure 3A). Once the first ice nuclei form at subzero temperatures, ice crystals grow until they reach an equilibrium with the remaining unfrozen fraction. As ice crystals form from pure water, the unfrozen fraction now contains a higher salt concentration and a lower freezing point. The cells remain in the channels of the unfrozen fraction [11]Mazur P. The role of intracellular freezing in the death of cells cooled at supraoptimal rates. Cryobiology 1977; 14: 251–72. Mazur P. The role of intracellular freezing in the death of cells cooled at supraoptimal rates. Cryobiology 1977; 14: 251–72. Mazur P. The role of intracellular freezing in the death of cells cooled at supraoptimal rates. Cryobiology 1977; 14: 251–72. , [12]Chen HH, Clarke DM, Gao D. Direct concentration measurements of the unfrozen portion of solutions under freezing. Cryobiology 2010; 61: 161–5. .
As freezing continues by reducing the temperature, more water solidifies out of the solution in the form of ice, resulting in increased salinity, solute toxicity, and increasingly lower freezing temperature of the remaining unfrozen fraction (Figure 3B & C).
The cells in the unfrozen fraction are then exposed to increasing salinity (and solute toxicity) as the temperature plunges (Figure 3D). At temperatures in the range below -20°C, the salinity of the unfrozen fraction may be up to 10–20 times the normothermic initial salinity. Recall that cell membranes become more permeable at lower temperatures. This increased salinity, and solute toxicity, impacts the intracellular milieu during freezing. Therefore, the magnitude of freezing-related stresses due to physical effectors (ice formation), and biochemical effectors (salinity, solute toxicity, protein structural damages, intracellular signals, etc.) is not insignificant. Furthermore, the cells respond osmotically to increased extracellular solute concentration by shrinking in size due to water efflux. Cells that are sensitive to these mechanical and biochemical changes are more likely to experience cell injury and cell death during freezing, including as freezing continues toward the glass transition temperature (Tg) of the cell-solution mixture, and then as vitrification into a glassy state occurs, under appropriate conditions [11]Mazur P. The role of intracellular freezing in the death of cells cooled at supraoptimal rates. Cryobiology 1977; 14: 251–72. Mazur P. The role of intracellular freezing in the death of cells cooled at supraoptimal rates. Cryobiology 1977; 14: 251–72. Mazur P. The role of intracellular freezing in the death of cells cooled at supraoptimal rates. Cryobiology 1977; 14: 251–72. .
The cell response to freezing
Now consider how a cell is affected by this freezing process, in the context of manufacturing a cell-based product: A slow freezing rate will allow the cells to respond osmotically to the ever-increasing osmolality of the extracellular milieu by losing water and shrinking in size (Figure 4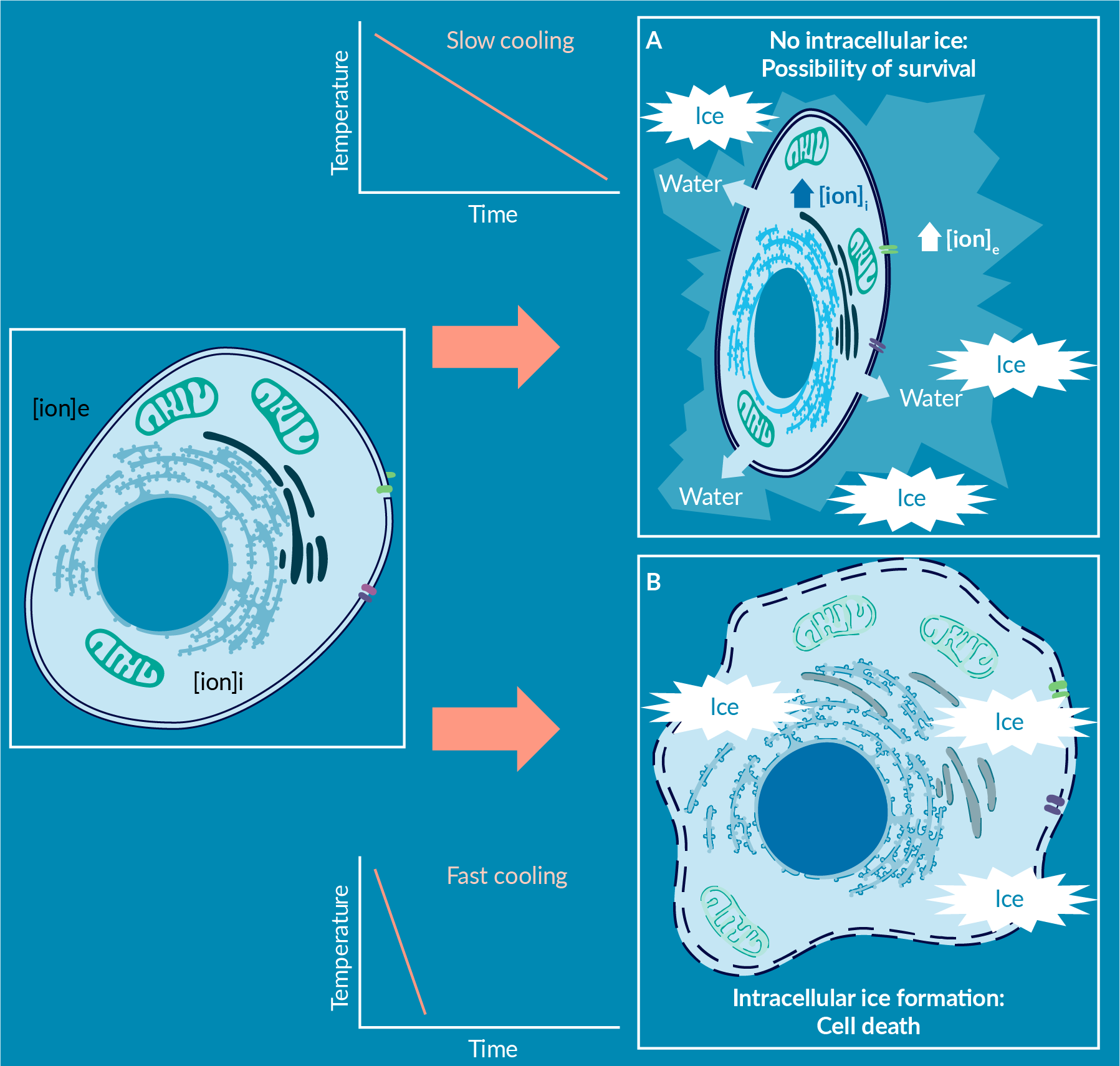
Osmotic shrinking, as a result of low temperatures and the cellular environment, is a dynamic process. As such, a fast freezing rate may not allow sufficient time for the cell to dehydrate enough water, and therefore increases the probability of intracellular ice formation [5]Hawkins BJ, Abazari A, Mathew AJ. Biopreservation Best Practices for regenerative medicine GMP manufacturing & focus on optimized biopreservation media. Cell Gene Ther. Ins. 2017; 3(5): 345–58. Hawkins BJ, Abazari A, Mathew AJ. Biopreservation Best Practices for regenerative medicine GMP manufacturing & focus on optimized biopreservation media. Cell Gene Ther. Ins. 2017; 3(5): 345–58. Hawkins BJ, Abazari A, Mathew AJ. Biopreservation Best Practices for regenerative medicine GMP manufacturing & focus on optimized biopreservation media. Cell Gene Ther. Ins. 2017; 3(5): 345–58. Hawkins BJ, Abazari A, Mathew AJ. Biopreservation Best Practices for regenerative medicine GMP manufacturing & focus on optimized biopreservation media. Cell Gene Ther. Ins. 2017; 3(5): 345–58. Hawkins BJ, Abazari A, Mathew AJ. Biopreservation Best Practices for regenerative medicine GMP manufacturing & focus on optimized biopreservation media. Cell Gene Ther. Ins. 2017; 3(5): 345–58. Hawkins BJ, Abazari A, Mathew AJ. Biopreservation Best Practices for regenerative medicine GMP manufacturing & focus on optimized biopreservation media. Cell Gene Ther. Ins. 2017; 3(5): 345–58. Hawkins BJ, Abazari A, Mathew AJ. Biopreservation Best Practices for regenerative medicine GMP manufacturing & focus on optimized biopreservation media. Cell Gene Ther. Ins. 2017; 3(5): 345–58. Hawkins BJ, Abazari A, Mathew AJ. Biopreservation Best Practices for regenerative medicine GMP manufacturing & focus on optimized biopreservation media. Cell Gene Ther. Ins. 2017; 3(5): 345–58. Hawkins BJ, Abazari A, Mathew AJ. Biopreservation Best Practices for regenerative medicine GMP manufacturing & focus on optimized biopreservation media. Cell Gene Ther. Ins. 2017; 3(5): 345–58. Hawkins BJ, Abazari A, Mathew AJ. Biopreservation Best Practices for regenerative medicine GMP manufacturing & focus on optimized biopreservation media. Cell Gene Ther. Ins. 2017; 3(5): 345–58. Hawkins BJ, Abazari A, Mathew AJ. Biopreservation Best Practices for regenerative medicine GMP manufacturing & focus on optimized biopreservation media. Cell Gene Ther. Ins. 2017; 3(5): 345–58. Hawkins BJ, Abazari A, Mathew AJ. Biopreservation Best Practices for regenerative medicine GMP manufacturing & focus on optimized biopreservation media. Cell Gene Ther. Ins. 2017; 3(5): 345–58. Hawkins BJ, Abazari A, Mathew AJ. Biopreservation Best Practices for regenerative medicine GMP manufacturing & focus on optimized biopreservation media. Cell Gene Ther. Ins. 2017; 3(5): 345–58. Hawkins BJ, Abazari A, Mathew AJ. Biopreservation Best Practices for regenerative medicine GMP manufacturing & focus on optimized biopreservation media. Cell Gene Ther. Ins. 2017; 3(5): 345–58. Hawkins BJ, Abazari A, Mathew AJ. Biopreservation Best Practices for regenerative medicine GMP manufacturing & focus on optimized biopreservation media. Cell Gene Ther. Ins. 2017; 3(5): 345–58. Hawkins BJ, Abazari A, Mathew AJ. Biopreservation Best Practices for regenerative medicine GMP manufacturing & focus on optimized biopreservation media. Cell Gene Ther. Ins. 2017; 3(5): 345–58. , [8]Abazari A. Process development considerations for cryopreservation of cellular therapies. Cell Gene Ther. Ins. 2019; 5(9): 1151–67.Abazari A. Process development considerations for cryopreservation of cellular therapies. Cell Gene Ther. Ins. 2019; 5(9): 1151–67.Abazari A. Process development considerations for cryopreservation of cellular therapies. Cell Gene Ther. Ins. 2019; 5(9): 1151–67.Abazari A. Process development considerations for cryopreservation of cellular therapies. Cell Gene Ther. Ins. 2019; 5(9): 1151–67.Abazari A. Process development considerations for cryopreservation of cellular therapies. Cell Gene Ther. Ins. 2019; 5(9): 1151–67.Abazari A. Process development considerations for cryopreservation of cellular therapies. Cell Gene Ther. Ins. 2019; 5(9): 1151–67.Abazari A. Process development considerations for cryopreservation of cellular therapies. Cell Gene Ther. Ins. 2019; 5(9): 1151–67.Abazari A. Process development considerations for cryopreservation of cellular therapies. Cell Gene Ther. Ins. 2019; 5(9): 1151–67.Abazari A. Process development considerations for cryopreservation of cellular therapies. Cell Gene Ther. Ins. 2019; 5(9): 1151–67., [9]Abazari A. Implementation of Biopreservation Best Practices to address a critical component of cell and gene therapy manufacturing: https://insights.bio/cell-and-gene-therapy-insights/implementation-of-biopreservation-best-practices-to-address-a-critical-component-of-cell-and-gene-therapy-manufacturing Abazari A. Implementation of Biopreservation Best Practices to address a critical component of cell and gene therapy manufacturing: https://insights.bio/cell-and-gene-therapy-insights/implementation-of-biopreservation-best-practices-to-address-a-critical-component-of-cell-and-gene-therapy-manufacturing Abazari A. Implementation of Biopreservation Best Practices to address a critical component of cell and gene therapy manufacturing: https://insights.bio/cell-and-gene-therapy-insights/implementation-of-biopreservation-best-practices-to-address-a-critical-component-of-cell-and-gene-therapy-manufacturing Abazari A. Implementation of Biopreservation Best Practices to address a critical component of cell and gene therapy manufacturing: https://insights.bio/cell-and-gene-therapy-insights/implementation-of-biopreservation-best-practices-to-address-a-critical-component-of-cell-and-gene-therapy-manufacturing Abazari A. Implementation of Biopreservation Best Practices to address a critical component of cell and gene therapy manufacturing: https://insights.bio/cell-and-gene-therapy-insights/implementation-of-biopreservation-best-practices-to-address-a-critical-component-of-cell-and-gene-therapy-manufacturing Abazari A. Implementation of Biopreservation Best Practices to address a critical component of cell and gene therapy manufacturing: https://insights.bio/cell-and-gene-therapy-insights/implementation-of-biopreservation-best-practices-to-address-a-critical-component-of-cell-and-gene-therapy-manufacturing Abazari A. Implementation of Biopreservation Best Practices to address a critical component of cell and gene therapy manufacturing: https://insights.bio/cell-and-gene-therapy-insights/implementation-of-biopreservation-best-practices-to-address-a-critical-component-of-cell-and-gene-therapy-manufacturing Abazari A. Implementation of Biopreservation Best Practices to address a critical component of cell and gene therapy manufacturing: https://insights.bio/cell-and-gene-therapy-insights/implementation-of-biopreservation-best-practices-to-address-a-critical-component-of-cell-and-gene-therapy-manufacturing Abazari A. Implementation of Biopreservation Best Practices to address a critical component of cell and gene therapy manufacturing: https://insights.bio/cell-and-gene-therapy-insights/implementation-of-biopreservation-best-practices-to-address-a-critical-component-of-cell-and-gene-therapy-manufacturing Abazari A. Implementation of Biopreservation Best Practices to address a critical component of cell and gene therapy manufacturing: https://insights.bio/cell-and-gene-therapy-insights/implementation-of-biopreservation-best-practices-to-address-a-critical-component-of-cell-and-gene-therapy-manufacturing Abazari A. Implementation of Biopreservation Best Practices to address a critical component of cell and gene therapy manufacturing: https://insights.bio/cell-and-gene-therapy-insights/implementation-of-biopreservation-best-practices-to-address-a-critical-component-of-cell-and-gene-therapy-manufacturing .
Growth of intracellular ice can physically rupture membranes. In the case of fast freezing rates, the cell may be lysed if the amount of ice is excessive, or may be damaged beyond repair even with lesser amounts of intracellular ice [5]Hawkins BJ, Abazari A, Mathew AJ. Biopreservation Best Practices for regenerative medicine GMP manufacturing & focus on optimized biopreservation media. Cell Gene Ther. Ins. 2017; 3(5): 345–58. Hawkins BJ, Abazari A, Mathew AJ. Biopreservation Best Practices for regenerative medicine GMP manufacturing & focus on optimized biopreservation media. Cell Gene Ther. Ins. 2017; 3(5): 345–58. Hawkins BJ, Abazari A, Mathew AJ. Biopreservation Best Practices for regenerative medicine GMP manufacturing & focus on optimized biopreservation media. Cell Gene Ther. Ins. 2017; 3(5): 345–58. Hawkins BJ, Abazari A, Mathew AJ. Biopreservation Best Practices for regenerative medicine GMP manufacturing & focus on optimized biopreservation media. Cell Gene Ther. Ins. 2017; 3(5): 345–58. Hawkins BJ, Abazari A, Mathew AJ. Biopreservation Best Practices for regenerative medicine GMP manufacturing & focus on optimized biopreservation media. Cell Gene Ther. Ins. 2017; 3(5): 345–58. Hawkins BJ, Abazari A, Mathew AJ. Biopreservation Best Practices for regenerative medicine GMP manufacturing & focus on optimized biopreservation media. Cell Gene Ther. Ins. 2017; 3(5): 345–58. Hawkins BJ, Abazari A, Mathew AJ. Biopreservation Best Practices for regenerative medicine GMP manufacturing & focus on optimized biopreservation media. Cell Gene Ther. Ins. 2017; 3(5): 345–58. Hawkins BJ, Abazari A, Mathew AJ. Biopreservation Best Practices for regenerative medicine GMP manufacturing & focus on optimized biopreservation media. Cell Gene Ther. Ins. 2017; 3(5): 345–58. Hawkins BJ, Abazari A, Mathew AJ. Biopreservation Best Practices for regenerative medicine GMP manufacturing & focus on optimized biopreservation media. Cell Gene Ther. Ins. 2017; 3(5): 345–58. Hawkins BJ, Abazari A, Mathew AJ. Biopreservation Best Practices for regenerative medicine GMP manufacturing & focus on optimized biopreservation media. Cell Gene Ther. Ins. 2017; 3(5): 345–58. Hawkins BJ, Abazari A, Mathew AJ. Biopreservation Best Practices for regenerative medicine GMP manufacturing & focus on optimized biopreservation media. Cell Gene Ther. Ins. 2017; 3(5): 345–58. Hawkins BJ, Abazari A, Mathew AJ. Biopreservation Best Practices for regenerative medicine GMP manufacturing & focus on optimized biopreservation media. Cell Gene Ther. Ins. 2017; 3(5): 345–58. Hawkins BJ, Abazari A, Mathew AJ. Biopreservation Best Practices for regenerative medicine GMP manufacturing & focus on optimized biopreservation media. Cell Gene Ther. Ins. 2017; 3(5): 345–58. Hawkins BJ, Abazari A, Mathew AJ. Biopreservation Best Practices for regenerative medicine GMP manufacturing & focus on optimized biopreservation media. Cell Gene Ther. Ins. 2017; 3(5): 345–58. Hawkins BJ, Abazari A, Mathew AJ. Biopreservation Best Practices for regenerative medicine GMP manufacturing & focus on optimized biopreservation media. Cell Gene Ther. Ins. 2017; 3(5): 345–58. Hawkins BJ, Abazari A, Mathew AJ. Biopreservation Best Practices for regenerative medicine GMP manufacturing & focus on optimized biopreservation media. Cell Gene Ther. Ins. 2017; 3(5): 345–58. , [8]Abazari A. Process development considerations for cryopreservation of cellular therapies. Cell Gene Ther. Ins. 2019; 5(9): 1151–67.Abazari A. Process development considerations for cryopreservation of cellular therapies. Cell Gene Ther. Ins. 2019; 5(9): 1151–67.Abazari A. Process development considerations for cryopreservation of cellular therapies. Cell Gene Ther. Ins. 2019; 5(9): 1151–67.Abazari A. Process development considerations for cryopreservation of cellular therapies. Cell Gene Ther. Ins. 2019; 5(9): 1151–67.Abazari A. Process development considerations for cryopreservation of cellular therapies. Cell Gene Ther. Ins. 2019; 5(9): 1151–67.Abazari A. Process development considerations for cryopreservation of cellular therapies. Cell Gene Ther. Ins. 2019; 5(9): 1151–67.Abazari A. Process development considerations for cryopreservation of cellular therapies. Cell Gene Ther. Ins. 2019; 5(9): 1151–67.Abazari A. Process development considerations for cryopreservation of cellular therapies. Cell Gene Ther. Ins. 2019; 5(9): 1151–67.Abazari A. Process development considerations for cryopreservation of cellular therapies. Cell Gene Ther. Ins. 2019; 5(9): 1151–67., [9]Abazari A. Implementation of Biopreservation Best Practices to address a critical component of cell and gene therapy manufacturing: https://insights.bio/cell-and-gene-therapy-insights/implementation-of-biopreservation-best-practices-to-address-a-critical-component-of-cell-and-gene-therapy-manufacturing Abazari A. Implementation of Biopreservation Best Practices to address a critical component of cell and gene therapy manufacturing: https://insights.bio/cell-and-gene-therapy-insights/implementation-of-biopreservation-best-practices-to-address-a-critical-component-of-cell-and-gene-therapy-manufacturing Abazari A. Implementation of Biopreservation Best Practices to address a critical component of cell and gene therapy manufacturing: https://insights.bio/cell-and-gene-therapy-insights/implementation-of-biopreservation-best-practices-to-address-a-critical-component-of-cell-and-gene-therapy-manufacturing Abazari A. Implementation of Biopreservation Best Practices to address a critical component of cell and gene therapy manufacturing: https://insights.bio/cell-and-gene-therapy-insights/implementation-of-biopreservation-best-practices-to-address-a-critical-component-of-cell-and-gene-therapy-manufacturing Abazari A. Implementation of Biopreservation Best Practices to address a critical component of cell and gene therapy manufacturing: https://insights.bio/cell-and-gene-therapy-insights/implementation-of-biopreservation-best-practices-to-address-a-critical-component-of-cell-and-gene-therapy-manufacturing Abazari A. Implementation of Biopreservation Best Practices to address a critical component of cell and gene therapy manufacturing: https://insights.bio/cell-and-gene-therapy-insights/implementation-of-biopreservation-best-practices-to-address-a-critical-component-of-cell-and-gene-therapy-manufacturing Abazari A. Implementation of Biopreservation Best Practices to address a critical component of cell and gene therapy manufacturing: https://insights.bio/cell-and-gene-therapy-insights/implementation-of-biopreservation-best-practices-to-address-a-critical-component-of-cell-and-gene-therapy-manufacturing Abazari A. Implementation of Biopreservation Best Practices to address a critical component of cell and gene therapy manufacturing: https://insights.bio/cell-and-gene-therapy-insights/implementation-of-biopreservation-best-practices-to-address-a-critical-component-of-cell-and-gene-therapy-manufacturing Abazari A. Implementation of Biopreservation Best Practices to address a critical component of cell and gene therapy manufacturing: https://insights.bio/cell-and-gene-therapy-insights/implementation-of-biopreservation-best-practices-to-address-a-critical-component-of-cell-and-gene-therapy-manufacturing Abazari A. Implementation of Biopreservation Best Practices to address a critical component of cell and gene therapy manufacturing: https://insights.bio/cell-and-gene-therapy-insights/implementation-of-biopreservation-best-practices-to-address-a-critical-component-of-cell-and-gene-therapy-manufacturing Abazari A. Implementation of Biopreservation Best Practices to address a critical component of cell and gene therapy manufacturing: https://insights.bio/cell-and-gene-therapy-insights/implementation-of-biopreservation-best-practices-to-address-a-critical-component-of-cell-and-gene-therapy-manufacturing .
In general, freezing rates around -1°C/min or so are observed to allow water-membrane dynamics to dehydrate CGT-relevant cell types sufficiently to reduce intracellular ice formation (Figure 5A). However, the level of osmotically-induced volume shrinkage may reach as low as 30% of the original cell volume. This may result in other forms of physical damage – including membrane folding and fusion, which is generally observed in the form of lower average cell volume, and an increase in the number of small non-cell vesicles post-thaw. The toxicity due to orders-of-magnitude increase in salinity, combined with mechanical cues from excessive osmotic shrinkage, induce adverse events in cells. These forms of cell damage and cell death include acute necrosis; and later Delayed Onset Cell Death (that becomes apparent as loss of viable recovery and function over hours to days post-thaw) [5]Hawkins BJ, Abazari A, Mathew AJ. Biopreservation Best Practices for regenerative medicine GMP manufacturing & focus on optimized biopreservation media. Cell Gene Ther. Ins. 2017; 3(5): 345–58. Hawkins BJ, Abazari A, Mathew AJ. Biopreservation Best Practices for regenerative medicine GMP manufacturing & focus on optimized biopreservation media. Cell Gene Ther. Ins. 2017; 3(5): 345–58. Hawkins BJ, Abazari A, Mathew AJ. Biopreservation Best Practices for regenerative medicine GMP manufacturing & focus on optimized biopreservation media. Cell Gene Ther. Ins. 2017; 3(5): 345–58. Hawkins BJ, Abazari A, Mathew AJ. Biopreservation Best Practices for regenerative medicine GMP manufacturing & focus on optimized biopreservation media. Cell Gene Ther. Ins. 2017; 3(5): 345–58. Hawkins BJ, Abazari A, Mathew AJ. Biopreservation Best Practices for regenerative medicine GMP manufacturing & focus on optimized biopreservation media. Cell Gene Ther. Ins. 2017; 3(5): 345–58. Hawkins BJ, Abazari A, Mathew AJ. Biopreservation Best Practices for regenerative medicine GMP manufacturing & focus on optimized biopreservation media. Cell Gene Ther. Ins. 2017; 3(5): 345–58. Hawkins BJ, Abazari A, Mathew AJ. Biopreservation Best Practices for regenerative medicine GMP manufacturing & focus on optimized biopreservation media. Cell Gene Ther. Ins. 2017; 3(5): 345–58. Hawkins BJ, Abazari A, Mathew AJ. Biopreservation Best Practices for regenerative medicine GMP manufacturing & focus on optimized biopreservation media. Cell Gene Ther. Ins. 2017; 3(5): 345–58. Hawkins BJ, Abazari A, Mathew AJ. Biopreservation Best Practices for regenerative medicine GMP manufacturing & focus on optimized biopreservation media. Cell Gene Ther. Ins. 2017; 3(5): 345–58. Hawkins BJ, Abazari A, Mathew AJ. Biopreservation Best Practices for regenerative medicine GMP manufacturing & focus on optimized biopreservation media. Cell Gene Ther. Ins. 2017; 3(5): 345–58. Hawkins BJ, Abazari A, Mathew AJ. Biopreservation Best Practices for regenerative medicine GMP manufacturing & focus on optimized biopreservation media. Cell Gene Ther. Ins. 2017; 3(5): 345–58. Hawkins BJ, Abazari A, Mathew AJ. Biopreservation Best Practices for regenerative medicine GMP manufacturing & focus on optimized biopreservation media. Cell Gene Ther. Ins. 2017; 3(5): 345–58. Hawkins BJ, Abazari A, Mathew AJ. Biopreservation Best Practices for regenerative medicine GMP manufacturing & focus on optimized biopreservation media. Cell Gene Ther. Ins. 2017; 3(5): 345–58. Hawkins BJ, Abazari A, Mathew AJ. Biopreservation Best Practices for regenerative medicine GMP manufacturing & focus on optimized biopreservation media. Cell Gene Ther. Ins. 2017; 3(5): 345–58. Hawkins BJ, Abazari A, Mathew AJ. Biopreservation Best Practices for regenerative medicine GMP manufacturing & focus on optimized biopreservation media. Cell Gene Ther. Ins. 2017; 3(5): 345–58. Hawkins BJ, Abazari A, Mathew AJ. Biopreservation Best Practices for regenerative medicine GMP manufacturing & focus on optimized biopreservation media. Cell Gene Ther. Ins. 2017; 3(5): 345–58. .
To reduce the osmotic shrinkage, and the toxicity due to increased solute concentration, cryoprotective agents (CPA) are added to the solution (membrane-permeable and/or non-permeating). One of the most well-known and most studied cryoprotective agents is dimethyl sulfoxide, or DMSO (Figure 5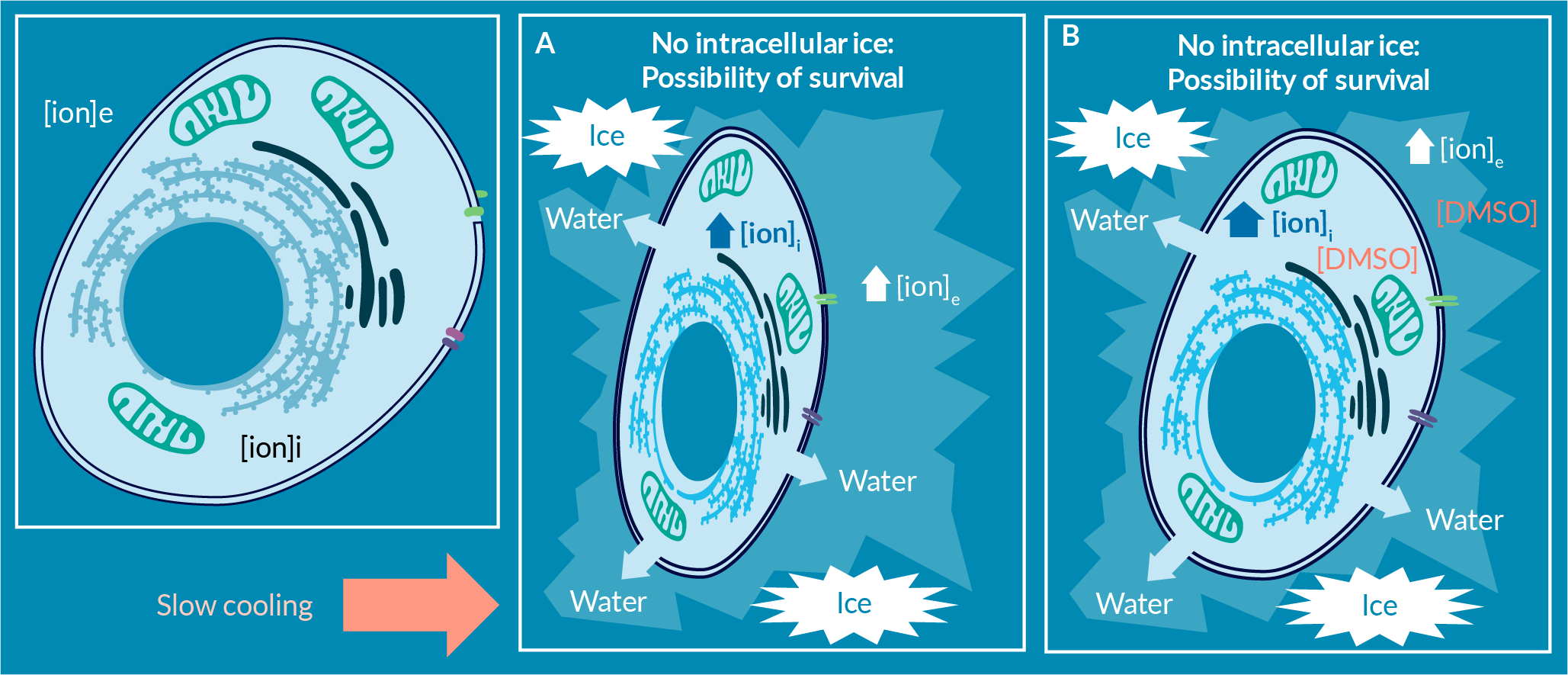
While referred to by some as an “anti-freeze” agent, DMSO offers protection against freezing in rather complex ways. In the unfrozen fraction, DMSO reduces salinity-induced toxicity and mechanical osmotic shrinkage by engaging water molecules and preventing ice crystal growth. As such, the cells are exposed to less salinity at any given temperature with the presence of DMSO. Furthermore, by permeating the cell, DMSO reduces the cell volumetric changes during freezing and minimizes intracellular ice growth [9]Abazari A. Implementation of Biopreservation Best Practices to address a critical component of cell and gene therapy manufacturing: https://insights.bio/cell-and-gene-therapy-insights/implementation-of-biopreservation-best-practices-to-address-a-critical-component-of-cell-and-gene-therapy-manufacturing Abazari A. Implementation of Biopreservation Best Practices to address a critical component of cell and gene therapy manufacturing: https://insights.bio/cell-and-gene-therapy-insights/implementation-of-biopreservation-best-practices-to-address-a-critical-component-of-cell-and-gene-therapy-manufacturing Abazari A. Implementation of Biopreservation Best Practices to address a critical component of cell and gene therapy manufacturing: https://insights.bio/cell-and-gene-therapy-insights/implementation-of-biopreservation-best-practices-to-address-a-critical-component-of-cell-and-gene-therapy-manufacturing Abazari A. Implementation of Biopreservation Best Practices to address a critical component of cell and gene therapy manufacturing: https://insights.bio/cell-and-gene-therapy-insights/implementation-of-biopreservation-best-practices-to-address-a-critical-component-of-cell-and-gene-therapy-manufacturing Abazari A. Implementation of Biopreservation Best Practices to address a critical component of cell and gene therapy manufacturing: https://insights.bio/cell-and-gene-therapy-insights/implementation-of-biopreservation-best-practices-to-address-a-critical-component-of-cell-and-gene-therapy-manufacturing Abazari A. Implementation of Biopreservation Best Practices to address a critical component of cell and gene therapy manufacturing: https://insights.bio/cell-and-gene-therapy-insights/implementation-of-biopreservation-best-practices-to-address-a-critical-component-of-cell-and-gene-therapy-manufacturing Abazari A. Implementation of Biopreservation Best Practices to address a critical component of cell and gene therapy manufacturing: https://insights.bio/cell-and-gene-therapy-insights/implementation-of-biopreservation-best-practices-to-address-a-critical-component-of-cell-and-gene-therapy-manufacturing Abazari A. Implementation of Biopreservation Best Practices to address a critical component of cell and gene therapy manufacturing: https://insights.bio/cell-and-gene-therapy-insights/implementation-of-biopreservation-best-practices-to-address-a-critical-component-of-cell-and-gene-therapy-manufacturing Abazari A. Implementation of Biopreservation Best Practices to address a critical component of cell and gene therapy manufacturing: https://insights.bio/cell-and-gene-therapy-insights/implementation-of-biopreservation-best-practices-to-address-a-critical-component-of-cell-and-gene-therapy-manufacturing Abazari A. Implementation of Biopreservation Best Practices to address a critical component of cell and gene therapy manufacturing: https://insights.bio/cell-and-gene-therapy-insights/implementation-of-biopreservation-best-practices-to-address-a-critical-component-of-cell-and-gene-therapy-manufacturing Abazari A. Implementation of Biopreservation Best Practices to address a critical component of cell and gene therapy manufacturing: https://insights.bio/cell-and-gene-therapy-insights/implementation-of-biopreservation-best-practices-to-address-a-critical-component-of-cell-and-gene-therapy-manufacturing . This particular set of actions of DMSO may not be readily replicated by other non-permeating cryoprotective agents and sugars, or other permeating cryoprotective agents with similar efficacy.
Why cryopreserve cell-based products?
Clinical and commercial manufacturing models drive several critical aspects about the CGT process and workflow. While, in theory, “fresh” non-frozen materials may be preferred by some (if even possible/feasible) due to simplicity (no cryopreservation step, no LN2 dewar shipping step, no thawing, no documentation for cryo-related procedures, etc.), the spatial separation biologistics of source starting materials/manufacturing activities/patients, and the globalization of supply chain management, are ameliorated by the temporal time management benefits of cryopreservation.
Living cells age, differentiate, and/or degrade over time, even under normothermic conditions. A reduction in temperature at strategic points in the CGT workflow reduces the biological activity and metabolic demands of cells, and slows down degradation. As temperatures decrease, metabolic and enzymatic activity slows, and at or below a glass transition temperature (Tg) of approximately -120°C to -130°C, molecular motion in water-based systems is virtually arrested [9]Abazari A. Implementation of Biopreservation Best Practices to address a critical component of cell and gene therapy manufacturing: https://insights.bio/cell-and-gene-therapy-insights/implementation-of-biopreservation-best-practices-to-address-a-critical-component-of-cell-and-gene-therapy-manufacturing Abazari A. Implementation of Biopreservation Best Practices to address a critical component of cell and gene therapy manufacturing: https://insights.bio/cell-and-gene-therapy-insights/implementation-of-biopreservation-best-practices-to-address-a-critical-component-of-cell-and-gene-therapy-manufacturing Abazari A. Implementation of Biopreservation Best Practices to address a critical component of cell and gene therapy manufacturing: https://insights.bio/cell-and-gene-therapy-insights/implementation-of-biopreservation-best-practices-to-address-a-critical-component-of-cell-and-gene-therapy-manufacturing Abazari A. Implementation of Biopreservation Best Practices to address a critical component of cell and gene therapy manufacturing: https://insights.bio/cell-and-gene-therapy-insights/implementation-of-biopreservation-best-practices-to-address-a-critical-component-of-cell-and-gene-therapy-manufacturing Abazari A. Implementation of Biopreservation Best Practices to address a critical component of cell and gene therapy manufacturing: https://insights.bio/cell-and-gene-therapy-insights/implementation-of-biopreservation-best-practices-to-address-a-critical-component-of-cell-and-gene-therapy-manufacturing Abazari A. Implementation of Biopreservation Best Practices to address a critical component of cell and gene therapy manufacturing: https://insights.bio/cell-and-gene-therapy-insights/implementation-of-biopreservation-best-practices-to-address-a-critical-component-of-cell-and-gene-therapy-manufacturing Abazari A. Implementation of Biopreservation Best Practices to address a critical component of cell and gene therapy manufacturing: https://insights.bio/cell-and-gene-therapy-insights/implementation-of-biopreservation-best-practices-to-address-a-critical-component-of-cell-and-gene-therapy-manufacturing Abazari A. Implementation of Biopreservation Best Practices to address a critical component of cell and gene therapy manufacturing: https://insights.bio/cell-and-gene-therapy-insights/implementation-of-biopreservation-best-practices-to-address-a-critical-component-of-cell-and-gene-therapy-manufacturing Abazari A. Implementation of Biopreservation Best Practices to address a critical component of cell and gene therapy manufacturing: https://insights.bio/cell-and-gene-therapy-insights/implementation-of-biopreservation-best-practices-to-address-a-critical-component-of-cell-and-gene-therapy-manufacturing Abazari A. Implementation of Biopreservation Best Practices to address a critical component of cell and gene therapy manufacturing: https://insights.bio/cell-and-gene-therapy-insights/implementation-of-biopreservation-best-practices-to-address-a-critical-component-of-cell-and-gene-therapy-manufacturing Abazari A. Implementation of Biopreservation Best Practices to address a critical component of cell and gene therapy manufacturing: https://insights.bio/cell-and-gene-therapy-insights/implementation-of-biopreservation-best-practices-to-address-a-critical-component-of-cell-and-gene-therapy-manufacturing . This vitrified state allows potential storage of the cell-based material for many years, and is a key temporal storage component of cell therapy manufacturing. An “investment” in cryopreservation buys time, provides flexibility, pays dividends through additional options, and is the most feasible current modality for long-term storage of CGT-related cell-based products.
Process development considerations for cryopreservation of cell-based therapies
Given the physics of freezing, and its effects on cells discussed above, it is important to determine if cryopreservation is appropriate and achievable for each CGT process/product. As developers of CGT therapies designed for successful commercial viability have looked to achieve a functional cryopreserved product, it is of value to understand that optimal cryopreservation of cells is not simply a matter of lowering the temperature below freezing. Some may think that cryopreservation consists of just freeze and thaw. However, the steps within a cryopreservation (and thaw) optimized method consists of multiple steps, with each step within the overall method potentially as a point of Risk and point of potential Optimization (Figure 6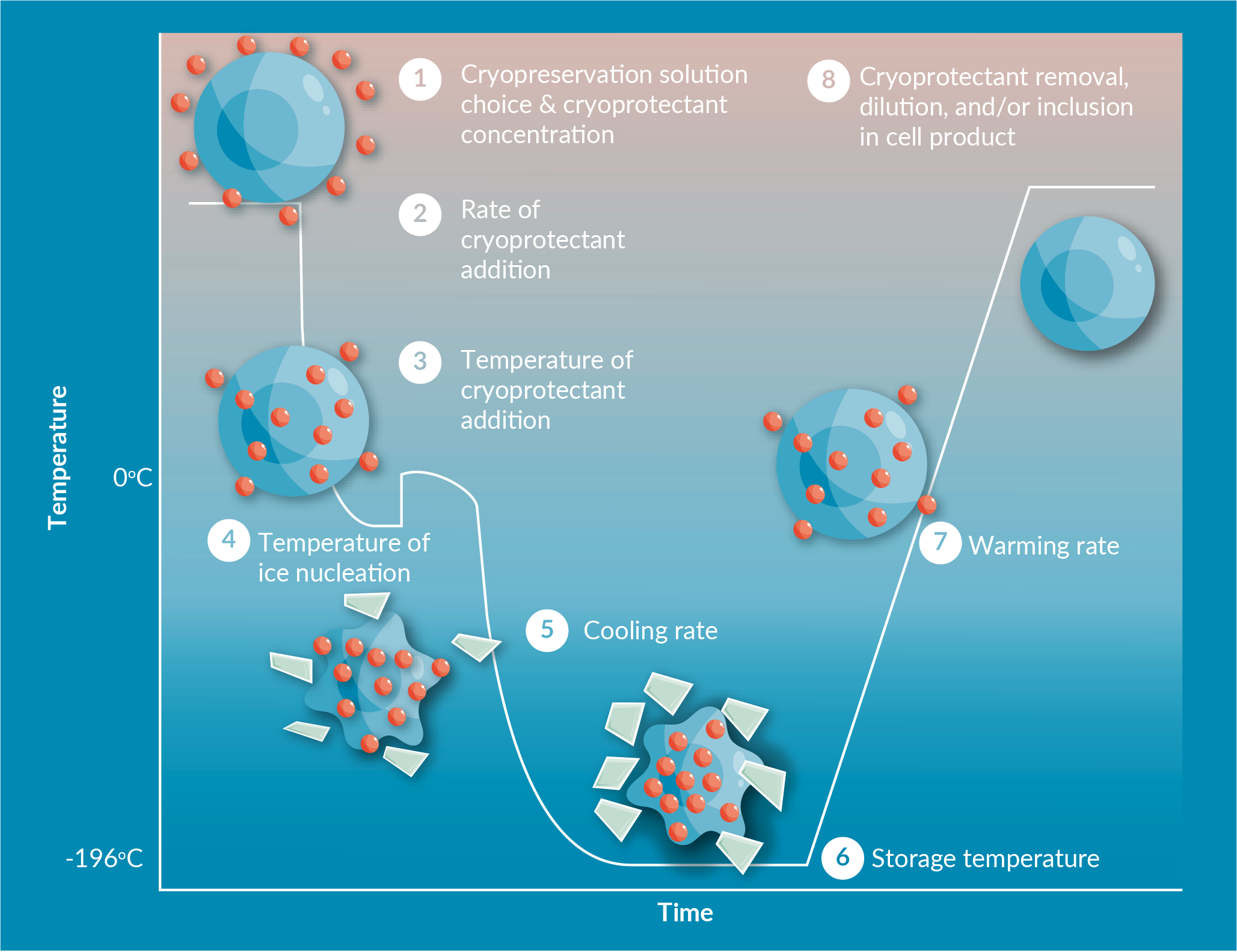
As illustrated in Figure 6, there are a number of steps within the cryopreservation method/protocol, that would be recommended to qualify/optimize from a Biopreservation Best Practices approach.
Consideration 1: Cryopreservation solution of choice. The traditional approach to the freeze media has been to formulate a home-brew cocktail of cryoprotectant (such as DMSO or glycerol), with serum (human or animal) or protein (albumin). These would be added to an isotonic (extracellular-like) vehicle solution such as culture media or saline-like solution, that had not been designed for low temperature biopreservation, but rather had been designed for normothermic ionic conditions. This formulation approach has been the traditional clinical center in-house home-brew cocktail, “grandfathered” into historical hematopoietic stem cell (HSC) transplant cryopreservation protocols [13]Berz D, McCormack EM, Winer ES et al. Cryopreservation of Hematopoietic Stem Cells. Am. J. Hematol. 2007; 82(6): 463–72., designed into some initial CGT cell therapies [14]KYMRIAH Prescribing Information, including Boxed WARNING, and Medication Guide: https://www.novartis.us/sites/www.novartis.us/files/kymriah.pdf, and even incorporated into some guiding standards (USP <1044> Cryopreservation of Cells) [15]USP <1044> Cryopreservation of Cells. Sept 27, 2018: https://www.usp.org/sites/default/files/usp/document/our-work/biologics/resources/gc-1044-cryopreservation-of-cells.pdf. In contrast, another more recent approach to the cryopreservation media has been to utilize a serum-free and protein-free intracellular-like formulation design, as discussed above [5]Hawkins BJ, Abazari A, Mathew AJ. Biopreservation Best Practices for regenerative medicine GMP manufacturing & focus on optimized biopreservation media. Cell Gene Ther. Ins. 2017; 3(5): 345–58. Hawkins BJ, Abazari A, Mathew AJ. Biopreservation Best Practices for regenerative medicine GMP manufacturing & focus on optimized biopreservation media. Cell Gene Ther. Ins. 2017; 3(5): 345–58. Hawkins BJ, Abazari A, Mathew AJ. Biopreservation Best Practices for regenerative medicine GMP manufacturing & focus on optimized biopreservation media. Cell Gene Ther. Ins. 2017; 3(5): 345–58. Hawkins BJ, Abazari A, Mathew AJ. Biopreservation Best Practices for regenerative medicine GMP manufacturing & focus on optimized biopreservation media. Cell Gene Ther. Ins. 2017; 3(5): 345–58. Hawkins BJ, Abazari A, Mathew AJ. Biopreservation Best Practices for regenerative medicine GMP manufacturing & focus on optimized biopreservation media. Cell Gene Ther. Ins. 2017; 3(5): 345–58. Hawkins BJ, Abazari A, Mathew AJ. Biopreservation Best Practices for regenerative medicine GMP manufacturing & focus on optimized biopreservation media. Cell Gene Ther. Ins. 2017; 3(5): 345–58. Hawkins BJ, Abazari A, Mathew AJ. Biopreservation Best Practices for regenerative medicine GMP manufacturing & focus on optimized biopreservation media. Cell Gene Ther. Ins. 2017; 3(5): 345–58. Hawkins BJ, Abazari A, Mathew AJ. Biopreservation Best Practices for regenerative medicine GMP manufacturing & focus on optimized biopreservation media. Cell Gene Ther. Ins. 2017; 3(5): 345–58. Hawkins BJ, Abazari A, Mathew AJ. Biopreservation Best Practices for regenerative medicine GMP manufacturing & focus on optimized biopreservation media. Cell Gene Ther. Ins. 2017; 3(5): 345–58. Hawkins BJ, Abazari A, Mathew AJ. Biopreservation Best Practices for regenerative medicine GMP manufacturing & focus on optimized biopreservation media. Cell Gene Ther. Ins. 2017; 3(5): 345–58. Hawkins BJ, Abazari A, Mathew AJ. Biopreservation Best Practices for regenerative medicine GMP manufacturing & focus on optimized biopreservation media. Cell Gene Ther. Ins. 2017; 3(5): 345–58. Hawkins BJ, Abazari A, Mathew AJ. Biopreservation Best Practices for regenerative medicine GMP manufacturing & focus on optimized biopreservation media. Cell Gene Ther. Ins. 2017; 3(5): 345–58. Hawkins BJ, Abazari A, Mathew AJ. Biopreservation Best Practices for regenerative medicine GMP manufacturing & focus on optimized biopreservation media. Cell Gene Ther. Ins. 2017; 3(5): 345–58. Hawkins BJ, Abazari A, Mathew AJ. Biopreservation Best Practices for regenerative medicine GMP manufacturing & focus on optimized biopreservation media. Cell Gene Ther. Ins. 2017; 3(5): 345–58. Hawkins BJ, Abazari A, Mathew AJ. Biopreservation Best Practices for regenerative medicine GMP manufacturing & focus on optimized biopreservation media. Cell Gene Ther. Ins. 2017; 3(5): 345–58. Hawkins BJ, Abazari A, Mathew AJ. Biopreservation Best Practices for regenerative medicine GMP manufacturing & focus on optimized biopreservation media. Cell Gene Ther. Ins. 2017; 3(5): 345–58. , [6]Abazari A, Hawkins BJ, Clarke DM, Mathew AJ. Biopreservation Best Practices: A Cornerstone in the Supply Chain of Cell-based Therapies – MSC Model Case Study. Cell Gene Ther. Ins. 2017; 3(10): 853–71. Abazari A, Hawkins BJ, Clarke DM, Mathew AJ. Biopreservation Best Practices: A Cornerstone in the Supply Chain of Cell-based Therapies – MSC Model Case Study. Cell Gene Ther. Ins. 2017; 3(10): 853–71. Abazari A, Hawkins BJ, Clarke DM, Mathew AJ. Biopreservation Best Practices: A Cornerstone in the Supply Chain of Cell-based Therapies – MSC Model Case Study. Cell Gene Ther. Ins. 2017; 3(10): 853–71. , [10]Mathew AJ. Biopreservation Considerations for Regenerative Medicine GMP Manufacturing; 2018: http://ebook.liebertpub.com/biolife-solutions/biopreservation-considerations-for-regenerative-medicine-gmp-manufacturing Mathew AJ. Biopreservation Considerations for Regenerative Medicine GMP Manufacturing; 2018: http://ebook.liebertpub.com/biolife-solutions/biopreservation-considerations-for-regenerative-medicine-gmp-manufacturing Mathew AJ. Biopreservation Considerations for Regenerative Medicine GMP Manufacturing; 2018: http://ebook.liebertpub.com/biolife-solutions/biopreservation-considerations-for-regenerative-medicine-gmp-manufacturing Mathew AJ. Biopreservation Considerations for Regenerative Medicine GMP Manufacturing; 2018: http://ebook.liebertpub.com/biolife-solutions/biopreservation-considerations-for-regenerative-medicine-gmp-manufacturing Mathew AJ. Biopreservation Considerations for Regenerative Medicine GMP Manufacturing; 2018: http://ebook.liebertpub.com/biolife-solutions/biopreservation-considerations-for-regenerative-medicine-gmp-manufacturing . This more recent methodology has been incorporated into many developing CGT, including ones that have obtained Regulatory clearances and Marketing Authorisations [16]YESCARTA Product Information – European Medicines Agency: https://www.ema.europa.eu/en/documents/product-information/yescarta-epar-product-information_en.pdf, [17]YESCARTA Product Monograph – Gilead Canada: http://www.gilead.ca/application/files/2715/8646/6805/Yescarta_English_PM_e214145-GS-002-Clean.pdf , [18]Zynteglo Product Information – European Medicines Agency: https://www.ema.europa.eu/en/documents/product-information/zynteglo-epar-product-information_en.pdf, [19]TECARTUS Package Insert – US FDA: https://www.fda.gov/media/140409/download .
Consideration 2: Rate of Cryoprotectant addition. Many research and clinical cryopreservation protocols proscribe slow/gradual/dropwise rates of addition of the cryoprotectant, in consideration to potential osmotic fluctuations and membrane permeability rates for the CPA. This consideration may, or may not, be impactful depending on the cell product/process. This consideration may also be less impactful with cryopreservation media that incorporate osmotic buffering components [20]Nicoud IA, Clarke DM, Taber G, Stolowski KM, Roberge SE, Song MK, Mathew AJ, Reems J. Cryopreservation of umbilical cord blood with a novel freezing solution that mimics intracellular ionic composition. Transfusion 2012; 52(9): 2055–62.Nicoud IA, Clarke DM, Taber G, Stolowski KM, Roberge SE, Song MK, Mathew AJ, Reems J. Cryopreservation of umbilical cord blood with a novel freezing solution that mimics intracellular ionic composition. Transfusion 2012; 52(9): 2055–62., [21]Lawson A, Mukherjee IN, Sambanis A. Mathematical modeling of cryoprotectant addition and removal for the cryopreservation of engineered or natural tissues. Cryobiology 2012; 64(1): 1–11.Lawson A, Mukherjee IN, Sambanis A. Mathematical modeling of cryoprotectant addition and removal for the cryopreservation of engineered or natural tissues. Cryobiology 2012; 64(1): 1–11., [22]Best BP. Cryoprotectant Toxicity: Facts, Issues, and Questions. Rejuvenation Res. 2015; 18(5): 422–36.Best BP. Cryoprotectant Toxicity: Facts, Issues, and Questions. Rejuvenation Res. 2015; 18(5): 422–36..
Consideration 3: Temperature of Cryoprotectant addition. Similar to the considerations related to the rate of CPA addition, some protocols proscribe a temperature for application of the freeze media. The choice of temperature may be related to facilitating more rapid permeability of the CPA, or related to reducing potential toxicity of the CPA [20]Nicoud IA, Clarke DM, Taber G, Stolowski KM, Roberge SE, Song MK, Mathew AJ, Reems J. Cryopreservation of umbilical cord blood with a novel freezing solution that mimics intracellular ionic composition. Transfusion 2012; 52(9): 2055–62.Nicoud IA, Clarke DM, Taber G, Stolowski KM, Roberge SE, Song MK, Mathew AJ, Reems J. Cryopreservation of umbilical cord blood with a novel freezing solution that mimics intracellular ionic composition. Transfusion 2012; 52(9): 2055–62., [21]Lawson A, Mukherjee IN, Sambanis A. Mathematical modeling of cryoprotectant addition and removal for the cryopreservation of engineered or natural tissues. Cryobiology 2012; 64(1): 1–11.Lawson A, Mukherjee IN, Sambanis A. Mathematical modeling of cryoprotectant addition and removal for the cryopreservation of engineered or natural tissues. Cryobiology 2012; 64(1): 1–11., [22]Best BP. Cryoprotectant Toxicity: Facts, Issues, and Questions. Rejuvenation Res. 2015; 18(5): 422–36.Best BP. Cryoprotectant Toxicity: Facts, Issues, and Questions. Rejuvenation Res. 2015; 18(5): 422–36..
Consideration 4: Temperature and consistency of ice nucleation. Some protocols may not speak to the point of ice nucleation within the cryopreservation procedure. Even with recognition of the ice nucleation, and related latent heat release, noted on freezing curves/graphs, there is often a passive approach to controlling ice nucleation within a method, let alone optimizing a method for consistent nucleation points from batch-to-batch of cell products. Lack of appropriate ice nucleation within a cryopreservation method may result in undercooling/supercooling of the sample, which may in turn be linked to deleterious intracellular ice formation and batch-to-batch variability. There are various approaches to the ice nucleation consideration [23]Morris GJ, Acton A. Controlled ice nucleation in cryopreservation – A review. Cryobiology 2013; 66: 85–92., and even approaches for method consistency with passive freezing devices [24]BioLife Solutions Cryopreservation Protocol: https://www.biolifesolutions.com/wp-content/uploads/2018/01/6012_07-CryoStor-Product-Information-Sheet.pdf . Programmable controlled rate freezers (CRF) are often utilized to provide consistent freezing rates and nucleation, however abnormal freezing curves and variable nucleation events may still occur and require troubleshooting [25]Creer MH, Mathew AJ, Lemas MV. Practical Handbook of Cellular Therapy Cryopreservation. AABB Press 2015. Creer MH, Mathew AJ, Lemas MV. Practical Handbook of Cellular Therapy Cryopreservation. AABB Press 2015. .
Consideration 5: Cooling rate. Although most CGT cell products might find cooling/freezing rates of approximately -1°C/min (averaged, or focused on the initial stage around nucleation) to be adequate, if not optimal [8]Abazari A. Process development considerations for cryopreservation of cellular therapies. Cell Gene Ther. Ins. 2019; 5(9): 1151–67.Abazari A. Process development considerations for cryopreservation of cellular therapies. Cell Gene Ther. Ins. 2019; 5(9): 1151–67.Abazari A. Process development considerations for cryopreservation of cellular therapies. Cell Gene Ther. Ins. 2019; 5(9): 1151–67.Abazari A. Process development considerations for cryopreservation of cellular therapies. Cell Gene Ther. Ins. 2019; 5(9): 1151–67.Abazari A. Process development considerations for cryopreservation of cellular therapies. Cell Gene Ther. Ins. 2019; 5(9): 1151–67.Abazari A. Process development considerations for cryopreservation of cellular therapies. Cell Gene Ther. Ins. 2019; 5(9): 1151–67.Abazari A. Process development considerations for cryopreservation of cellular therapies. Cell Gene Ther. Ins. 2019; 5(9): 1151–67.Abazari A. Process development considerations for cryopreservation of cellular therapies. Cell Gene Ther. Ins. 2019; 5(9): 1151–67.Abazari A. Process development considerations for cryopreservation of cellular therapies. Cell Gene Ther. Ins. 2019; 5(9): 1151–67., [9]Abazari A. Implementation of Biopreservation Best Practices to address a critical component of cell and gene therapy manufacturing: https://insights.bio/cell-and-gene-therapy-insights/implementation-of-biopreservation-best-practices-to-address-a-critical-component-of-cell-and-gene-therapy-manufacturing Abazari A. Implementation of Biopreservation Best Practices to address a critical component of cell and gene therapy manufacturing: https://insights.bio/cell-and-gene-therapy-insights/implementation-of-biopreservation-best-practices-to-address-a-critical-component-of-cell-and-gene-therapy-manufacturing Abazari A. Implementation of Biopreservation Best Practices to address a critical component of cell and gene therapy manufacturing: https://insights.bio/cell-and-gene-therapy-insights/implementation-of-biopreservation-best-practices-to-address-a-critical-component-of-cell-and-gene-therapy-manufacturing Abazari A. Implementation of Biopreservation Best Practices to address a critical component of cell and gene therapy manufacturing: https://insights.bio/cell-and-gene-therapy-insights/implementation-of-biopreservation-best-practices-to-address-a-critical-component-of-cell-and-gene-therapy-manufacturing Abazari A. Implementation of Biopreservation Best Practices to address a critical component of cell and gene therapy manufacturing: https://insights.bio/cell-and-gene-therapy-insights/implementation-of-biopreservation-best-practices-to-address-a-critical-component-of-cell-and-gene-therapy-manufacturing Abazari A. Implementation of Biopreservation Best Practices to address a critical component of cell and gene therapy manufacturing: https://insights.bio/cell-and-gene-therapy-insights/implementation-of-biopreservation-best-practices-to-address-a-critical-component-of-cell-and-gene-therapy-manufacturing Abazari A. Implementation of Biopreservation Best Practices to address a critical component of cell and gene therapy manufacturing: https://insights.bio/cell-and-gene-therapy-insights/implementation-of-biopreservation-best-practices-to-address-a-critical-component-of-cell-and-gene-therapy-manufacturing Abazari A. Implementation of Biopreservation Best Practices to address a critical component of cell and gene therapy manufacturing: https://insights.bio/cell-and-gene-therapy-insights/implementation-of-biopreservation-best-practices-to-address-a-critical-component-of-cell-and-gene-therapy-manufacturing Abazari A. Implementation of Biopreservation Best Practices to address a critical component of cell and gene therapy manufacturing: https://insights.bio/cell-and-gene-therapy-insights/implementation-of-biopreservation-best-practices-to-address-a-critical-component-of-cell-and-gene-therapy-manufacturing Abazari A. Implementation of Biopreservation Best Practices to address a critical component of cell and gene therapy manufacturing: https://insights.bio/cell-and-gene-therapy-insights/implementation-of-biopreservation-best-practices-to-address-a-critical-component-of-cell-and-gene-therapy-manufacturing Abazari A. Implementation of Biopreservation Best Practices to address a critical component of cell and gene therapy manufacturing: https://insights.bio/cell-and-gene-therapy-insights/implementation-of-biopreservation-best-practices-to-address-a-critical-component-of-cell-and-gene-therapy-manufacturing , [11]Mazur P. The role of intracellular freezing in the death of cells cooled at supraoptimal rates. Cryobiology 1977; 14: 251–72. Mazur P. The role of intracellular freezing in the death of cells cooled at supraoptimal rates. Cryobiology 1977; 14: 251–72. Mazur P. The role of intracellular freezing in the death of cells cooled at supraoptimal rates. Cryobiology 1977; 14: 251–72. , [26]Baboo J, Kilbride P, Delahaye M et al. The Impact of Varying Cooling and Thawing Rates on the Quality of Cryopreserved Human Peripheral Blood T Cells. Sci. Rep. 2019; 9: 3417. Baboo J, Kilbride P, Delahaye M et al. The Impact of Varying Cooling and Thawing Rates on the Quality of Cryopreserved Human Peripheral Blood T Cells. Sci. Rep. 2019; 9: 3417. , it would be recommended (and often expected) to verify, and perhaps optimize, the freezing rates as appropriate for each manufactured cell product as an evidence-based Biopreservation Best Practice. Even with use of a programmable CRF, the stages within the CRF program may be optimized for various cell product parameters (cell type, cell volume, membrane permeability, cell concentration, product volume, product packaging, number of product units, etc.). CRF abnormal freezing curves may still occur and require troubleshooting [25]Creer MH, Mathew AJ, Lemas MV. Practical Handbook of Cellular Therapy Cryopreservation. AABB Press 2015. Creer MH, Mathew AJ, Lemas MV. Practical Handbook of Cellular Therapy Cryopreservation. AABB Press 2015. .
Consideration 6: Storage temperature. Cryopreserved CGT products are generally stored in liquid nitrogen (LN2), to facilitate ultra-low cryogenic temperatures below their glass transition (Tg) temperature, and to enable many years of stability [27]Meneghel J, Kilbride P, Morris GJ et al. Physical events occurring during the cryopreservation of immortalized human T cells. PLoS ONE 2019; 14(5).. Alternatively, there may be potential for further consideration of shorter-term stability (weeks to months) at temperatures in the range of -80°C. The feasibility of varying storage temperatures (and the related pros and cons) may be worth exploring, and may be able to support short-term storage aligned with less burdensome storage/transport needs, with more robust cryopreservation methods and cold chain management[28]Kofanova OA, Davis K, Glazer B et al. Viable Mononuclear Cell Stability Study for Implementation in a Proficiency Testing Program: Impact of Shipment Conditions. Biopreserv. Biobank. 2014; 12(3): 206–16. , [29]Abazari A, Hawkins BJ, Fink J, O’Donnell K, Mathew AJ. Next Generation Technology, Procedures, and Products Facilitate Biopreservation Best Practices for Cellular Therapies. 2016: https://www.biolifesolutions.com/wp-content/uploads/2016/10/Biolife_Brooks_Whitepaper_OCT20_REL.pdf.
Consideration 7: Warming/Thawing rate. In alignment with most CGT slow-freeze cryopreservation protocols, the most common thawing methods for those cryopreserved cell products involve fast-thaw methods with traditional 37°C waterbaths. At a superficial level, the process mirrors that of freezing: warming of the sample from cryogenic temperatures toward the solid-to-liquid phase transition, melting of ice to form liquid water, and rehydration of the cells. Similar to historical cryopreservation methods, this method of fast thawing has been largely adequate. The criticality of thawing rates is a noted point of discussion [26]Baboo J, Kilbride P, Delahaye M et al. The Impact of Varying Cooling and Thawing Rates on the Quality of Cryopreserved Human Peripheral Blood T Cells. Sci. Rep. 2019; 9: 3417. Baboo J, Kilbride P, Delahaye M et al. The Impact of Varying Cooling and Thawing Rates on the Quality of Cryopreserved Human Peripheral Blood T Cells. Sci. Rep. 2019; 9: 3417. , and thaw methods (including rate of thawing) would be a worthwhile process parameter to investigate and verify for each cell product/process with an evidence-based approach to asses Risk and potential Optimization [8]Abazari A. Process development considerations for cryopreservation of cellular therapies. Cell Gene Ther. Ins. 2019; 5(9): 1151–67.Abazari A. Process development considerations for cryopreservation of cellular therapies. Cell Gene Ther. Ins. 2019; 5(9): 1151–67.Abazari A. Process development considerations for cryopreservation of cellular therapies. Cell Gene Ther. Ins. 2019; 5(9): 1151–67.Abazari A. Process development considerations for cryopreservation of cellular therapies. Cell Gene Ther. Ins. 2019; 5(9): 1151–67.Abazari A. Process development considerations for cryopreservation of cellular therapies. Cell Gene Ther. Ins. 2019; 5(9): 1151–67.Abazari A. Process development considerations for cryopreservation of cellular therapies. Cell Gene Ther. Ins. 2019; 5(9): 1151–67.Abazari A. Process development considerations for cryopreservation of cellular therapies. Cell Gene Ther. Ins. 2019; 5(9): 1151–67.Abazari A. Process development considerations for cryopreservation of cellular therapies. Cell Gene Ther. Ins. 2019; 5(9): 1151–67.Abazari A. Process development considerations for cryopreservation of cellular therapies. Cell Gene Ther. Ins. 2019; 5(9): 1151–67., [9]Abazari A. Implementation of Biopreservation Best Practices to address a critical component of cell and gene therapy manufacturing: https://insights.bio/cell-and-gene-therapy-insights/implementation-of-biopreservation-best-practices-to-address-a-critical-component-of-cell-and-gene-therapy-manufacturing Abazari A. Implementation of Biopreservation Best Practices to address a critical component of cell and gene therapy manufacturing: https://insights.bio/cell-and-gene-therapy-insights/implementation-of-biopreservation-best-practices-to-address-a-critical-component-of-cell-and-gene-therapy-manufacturing Abazari A. Implementation of Biopreservation Best Practices to address a critical component of cell and gene therapy manufacturing: https://insights.bio/cell-and-gene-therapy-insights/implementation-of-biopreservation-best-practices-to-address-a-critical-component-of-cell-and-gene-therapy-manufacturing Abazari A. Implementation of Biopreservation Best Practices to address a critical component of cell and gene therapy manufacturing: https://insights.bio/cell-and-gene-therapy-insights/implementation-of-biopreservation-best-practices-to-address-a-critical-component-of-cell-and-gene-therapy-manufacturing Abazari A. Implementation of Biopreservation Best Practices to address a critical component of cell and gene therapy manufacturing: https://insights.bio/cell-and-gene-therapy-insights/implementation-of-biopreservation-best-practices-to-address-a-critical-component-of-cell-and-gene-therapy-manufacturing Abazari A. Implementation of Biopreservation Best Practices to address a critical component of cell and gene therapy manufacturing: https://insights.bio/cell-and-gene-therapy-insights/implementation-of-biopreservation-best-practices-to-address-a-critical-component-of-cell-and-gene-therapy-manufacturing Abazari A. Implementation of Biopreservation Best Practices to address a critical component of cell and gene therapy manufacturing: https://insights.bio/cell-and-gene-therapy-insights/implementation-of-biopreservation-best-practices-to-address-a-critical-component-of-cell-and-gene-therapy-manufacturing Abazari A. Implementation of Biopreservation Best Practices to address a critical component of cell and gene therapy manufacturing: https://insights.bio/cell-and-gene-therapy-insights/implementation-of-biopreservation-best-practices-to-address-a-critical-component-of-cell-and-gene-therapy-manufacturing Abazari A. Implementation of Biopreservation Best Practices to address a critical component of cell and gene therapy manufacturing: https://insights.bio/cell-and-gene-therapy-insights/implementation-of-biopreservation-best-practices-to-address-a-critical-component-of-cell-and-gene-therapy-manufacturing Abazari A. Implementation of Biopreservation Best Practices to address a critical component of cell and gene therapy manufacturing: https://insights.bio/cell-and-gene-therapy-insights/implementation-of-biopreservation-best-practices-to-address-a-critical-component-of-cell-and-gene-therapy-manufacturing Abazari A. Implementation of Biopreservation Best Practices to address a critical component of cell and gene therapy manufacturing: https://insights.bio/cell-and-gene-therapy-insights/implementation-of-biopreservation-best-practices-to-address-a-critical-component-of-cell-and-gene-therapy-manufacturing .
Consideration 8: Post-thaw wash, dilution, or direct application. There are a variety of approaches (and dogma) regarding the post-thaw status of the cryopreservation medium. One school of thought is that the cryoprotectant(s) must be removed post-thaw. The CPA removal might be via a single step wash/centrifugation, or via stepwise dilution and wash in consideration to osmotic fluctuations. There has also been development and application of various washing devices. Another approach would be to dilute post-thaw, but not wash/remove the CPA in entirety. And then there is the approach of avoiding wash or dilution with direct post-thaw application. Each of those approaches has potential benefits and drawbacks, that might range from extensive cell damage/loss (wash and removal methods) to potential (or perceived) cryoprotectant toxicity (direct application). Each approach also entails a different level of post-thaw manipulation, and potential variability at the point of post-thaw application [5]Hawkins BJ, Abazari A, Mathew AJ. Biopreservation Best Practices for regenerative medicine GMP manufacturing & focus on optimized biopreservation media. Cell Gene Ther. Ins. 2017; 3(5): 345–58. Hawkins BJ, Abazari A, Mathew AJ. Biopreservation Best Practices for regenerative medicine GMP manufacturing & focus on optimized biopreservation media. Cell Gene Ther. Ins. 2017; 3(5): 345–58. Hawkins BJ, Abazari A, Mathew AJ. Biopreservation Best Practices for regenerative medicine GMP manufacturing & focus on optimized biopreservation media. Cell Gene Ther. Ins. 2017; 3(5): 345–58. Hawkins BJ, Abazari A, Mathew AJ. Biopreservation Best Practices for regenerative medicine GMP manufacturing & focus on optimized biopreservation media. Cell Gene Ther. Ins. 2017; 3(5): 345–58. Hawkins BJ, Abazari A, Mathew AJ. Biopreservation Best Practices for regenerative medicine GMP manufacturing & focus on optimized biopreservation media. Cell Gene Ther. Ins. 2017; 3(5): 345–58. Hawkins BJ, Abazari A, Mathew AJ. Biopreservation Best Practices for regenerative medicine GMP manufacturing & focus on optimized biopreservation media. Cell Gene Ther. Ins. 2017; 3(5): 345–58. Hawkins BJ, Abazari A, Mathew AJ. Biopreservation Best Practices for regenerative medicine GMP manufacturing & focus on optimized biopreservation media. Cell Gene Ther. Ins. 2017; 3(5): 345–58. Hawkins BJ, Abazari A, Mathew AJ. Biopreservation Best Practices for regenerative medicine GMP manufacturing & focus on optimized biopreservation media. Cell Gene Ther. Ins. 2017; 3(5): 345–58. Hawkins BJ, Abazari A, Mathew AJ. Biopreservation Best Practices for regenerative medicine GMP manufacturing & focus on optimized biopreservation media. Cell Gene Ther. Ins. 2017; 3(5): 345–58. Hawkins BJ, Abazari A, Mathew AJ. Biopreservation Best Practices for regenerative medicine GMP manufacturing & focus on optimized biopreservation media. Cell Gene Ther. Ins. 2017; 3(5): 345–58. Hawkins BJ, Abazari A, Mathew AJ. Biopreservation Best Practices for regenerative medicine GMP manufacturing & focus on optimized biopreservation media. Cell Gene Ther. Ins. 2017; 3(5): 345–58. Hawkins BJ, Abazari A, Mathew AJ. Biopreservation Best Practices for regenerative medicine GMP manufacturing & focus on optimized biopreservation media. Cell Gene Ther. Ins. 2017; 3(5): 345–58. Hawkins BJ, Abazari A, Mathew AJ. Biopreservation Best Practices for regenerative medicine GMP manufacturing & focus on optimized biopreservation media. Cell Gene Ther. Ins. 2017; 3(5): 345–58. Hawkins BJ, Abazari A, Mathew AJ. Biopreservation Best Practices for regenerative medicine GMP manufacturing & focus on optimized biopreservation media. Cell Gene Ther. Ins. 2017; 3(5): 345–58. Hawkins BJ, Abazari A, Mathew AJ. Biopreservation Best Practices for regenerative medicine GMP manufacturing & focus on optimized biopreservation media. Cell Gene Ther. Ins. 2017; 3(5): 345–58. Hawkins BJ, Abazari A, Mathew AJ. Biopreservation Best Practices for regenerative medicine GMP manufacturing & focus on optimized biopreservation media. Cell Gene Ther. Ins. 2017; 3(5): 345–58. , [8]Abazari A. Process development considerations for cryopreservation of cellular therapies. Cell Gene Ther. Ins. 2019; 5(9): 1151–67.Abazari A. Process development considerations for cryopreservation of cellular therapies. Cell Gene Ther. Ins. 2019; 5(9): 1151–67.Abazari A. Process development considerations for cryopreservation of cellular therapies. Cell Gene Ther. Ins. 2019; 5(9): 1151–67.Abazari A. Process development considerations for cryopreservation of cellular therapies. Cell Gene Ther. Ins. 2019; 5(9): 1151–67.Abazari A. Process development considerations for cryopreservation of cellular therapies. Cell Gene Ther. Ins. 2019; 5(9): 1151–67.Abazari A. Process development considerations for cryopreservation of cellular therapies. Cell Gene Ther. Ins. 2019; 5(9): 1151–67.Abazari A. Process development considerations for cryopreservation of cellular therapies. Cell Gene Ther. Ins. 2019; 5(9): 1151–67.Abazari A. Process development considerations for cryopreservation of cellular therapies. Cell Gene Ther. Ins. 2019; 5(9): 1151–67.Abazari A. Process development considerations for cryopreservation of cellular therapies. Cell Gene Ther. Ins. 2019; 5(9): 1151–67., [10]Mathew AJ. Biopreservation Considerations for Regenerative Medicine GMP Manufacturing; 2018: http://ebook.liebertpub.com/biolife-solutions/biopreservation-considerations-for-regenerative-medicine-gmp-manufacturing Mathew AJ. Biopreservation Considerations for Regenerative Medicine GMP Manufacturing; 2018: http://ebook.liebertpub.com/biolife-solutions/biopreservation-considerations-for-regenerative-medicine-gmp-manufacturing Mathew AJ. Biopreservation Considerations for Regenerative Medicine GMP Manufacturing; 2018: http://ebook.liebertpub.com/biolife-solutions/biopreservation-considerations-for-regenerative-medicine-gmp-manufacturing Mathew AJ. Biopreservation Considerations for Regenerative Medicine GMP Manufacturing; 2018: http://ebook.liebertpub.com/biolife-solutions/biopreservation-considerations-for-regenerative-medicine-gmp-manufacturing Mathew AJ. Biopreservation Considerations for Regenerative Medicine GMP Manufacturing; 2018: http://ebook.liebertpub.com/biolife-solutions/biopreservation-considerations-for-regenerative-medicine-gmp-manufacturing , [30]Awan M, Buriak I, Fleck R et al. Dimethyl sulfoxide: a central player since the dawn of cryobiology, is efficacy balanced by toxicity? Regen. Med. 2020; 15(3): 1463–91. .
Biopreservation best practices considerations
Most evidence-based best practices identify the process parameters, and investigate the characteristics that can impact the critical quality attributes of the product. Within the considerations of biopreservation, broader process best practices may overlap to more focused Biopreservation Best Practices that can serve as a guiding approach applicable to CGT manufacturing (Figure 7
Often, the early-stage development of a product understandably focuses on the high-level product efficacy (recovery, viability, and perhaps some measure of functionality). Admittedly, if the feasibility of that aspect is not established, the other parameters may be moot considerations. The ability to manufacture the product tends to be an early translational focus, and as the product progresses along potential clinical or commercial development there is increasing scrutiny to Quality and/or Regulatory Risk considerations. Areas of overlap with focus on Biopreservation Best Practices may include:
- Ability to integrate a biopreservation tool (media, equipment, method, etc.) into the CGT manufacturing process, including risk from process change.
- Cost-effectiveness of those tools and technologies, such as pre-formulated biopreservation media or controlled rate freezer.
- Efficacy of the tools, methods, and cell product.
- Impact to Quality and Regulatory footprint, such as safety of biopreservation media and consideration to qualification for excipient application. Also, consideration to alignment with Good Manufacturing Practices (GMP).
- Qualification and validation of the tools, technologies, or methods.
- Supplier reliability, risk, expertise, and qualification alignment. Also, supply chain security of the tools and technologies.
Additional biopreservation process parameters
As an extension of the number of critical steps within the cryopreservation process (Figure 6), there are Biopreservation Critical Process Parameters (BCPP) throughout the CGT manufacturing process, and including where biopreservation and stability might impact the quality attributes of the process/product (Figure 8
Cold chain management
Advances have been made in cold chain management systems, and monitoring of this critical part of the CGT workflow. Innovations in insulating materials have overcome shortcomings in insulated packaging performance. ‘SMART’ shippers with improved cloud-based data tracking and software technology have enhanced management of time-critical and temperature-sensitive products. Technology innovations have improved packaging, monitoring, logistics practices, data collection and data management; and incorporated them into unique, innovative, and self-contained systems [31]O’Donnell K. Moving from passive to rescue design packaging: helping cells arrive alive with smart shippers. Cell Gene Ther. Ins. 2015; 1(2): 163–71., [32]O’Donnell K, Mathew AJ. Cell and Gene Therapies in Transit: Caution – Hazards Ahead. Cell Gene Ther. Ins. 2016: https://www.insights.bio/cell-and-gene-therapy-insights/journal/article/474/Cell-and-Gene-Therapies-in-Transit-Caution-Hazards-Ahead. , [33]United Airlines Cargo, SAVSU. Transforming the future of medical shipments. 2019: https://ual.unitedcargo.com/Transforming-the-future-of-medical-shipments-SOCIAL-Page. United Airlines Cargo, SAVSU. Transforming the future of medical shipments. 2019: https://ual.unitedcargo.com/Transforming-the-future-of-medical-shipments-SOCIAL-Page. United Airlines Cargo, SAVSU. Transforming the future of medical shipments. 2019: https://ual.unitedcargo.com/Transforming-the-future-of-medical-shipments-SOCIAL-Page. .
SMART cold chain technologies such as Liquid Nitrogen (LN2) “dry vapor” SMART shippers and longer-range dry ice shippers are increasingly being utilized by late-stage clinical trial and commercialized therapy providers. The temperature monitoring and control, location tracking, chain of custody monitoring, and long temperature life of these shippers addresses a critical part of the supply chain biologistics [33]United Airlines Cargo, SAVSU. Transforming the future of medical shipments. 2019: https://ual.unitedcargo.com/Transforming-the-future-of-medical-shipments-SOCIAL-Page. United Airlines Cargo, SAVSU. Transforming the future of medical shipments. 2019: https://ual.unitedcargo.com/Transforming-the-future-of-medical-shipments-SOCIAL-Page. United Airlines Cargo, SAVSU. Transforming the future of medical shipments. 2019: https://ual.unitedcargo.com/Transforming-the-future-of-medical-shipments-SOCIAL-Page. . With LN2 shippers, traditional LN2 dry vapor shippers experience reduced performance when not maintained upright, they may require palletization, and therefore may be restricted to wide-body aircraft and limited to large airport channels. New shipper technologies look to maintain temperature under some tilting, accommodate loading onto smaller regional aircraft that cannot support palletized cargo, and enable greater flexibility during transport [33]United Airlines Cargo, SAVSU. Transforming the future of medical shipments. 2019: https://ual.unitedcargo.com/Transforming-the-future-of-medical-shipments-SOCIAL-Page. United Airlines Cargo, SAVSU. Transforming the future of medical shipments. 2019: https://ual.unitedcargo.com/Transforming-the-future-of-medical-shipments-SOCIAL-Page. United Airlines Cargo, SAVSU. Transforming the future of medical shipments. 2019: https://ual.unitedcargo.com/Transforming-the-future-of-medical-shipments-SOCIAL-Page. .
Thawing
In order to transition from cryopreserved samples/product to application of the cells, the intermediate step is returning cell samples/products to the non-frozen state. Optimal thawing of these cells may be critical to successful downstream applications. Thawing rate and temperature may be parameters for potential optimization for cell size and volume, cell type, and cryopreservation media.
The most common and well-accepted method for rapidly thawing cryopreserved cell samples is partial submersion of the sample in a 37°C waterbath. There are several reasons for using this approach: waterbaths are relatively cheap and easily available, and they allow efficient heat transfer from the water to the sample due to the high heat capacity and thermal conductivity of liquid water. However, there are potential risks to using a waterbath for thawing, particularly in a clinical environment. These potential risks include:
- Lack of scalability post-manufacturing.
- User-to-user variability in subjectively determining thaw recognition times,
final vial temperature, and ending point of ice. - Overthawing, or excessive warming, of samples.
- No data management or chain-of-custody connectivity.
- Contamination of sample contents.
- Challenge in using a waterbath as part of a sterile process inside a biosafety cabinet or clean environment.
- Restrictions in using waterbaths in GMP or clinical environments.
To overcome some of the limitations of using waterbaths for thawing, researchers and process engineers have explored other options such as dry bead baths or heat blocks [34]Röllig C, Babatz J, Wagner I et al. Thawing of cryopreserved mobilized peripheral blood--comparison between waterbath and dry warming device. Cytotherapy 2002; 4(6): 551–5., [35]Triana E, Ortega S, Azqueta C et al. Thawing of cryopreserved hematopoietic progenitor cells from apheresis with a new dry-warming device. Transfusion 2013; 53(1): 85–90. . Unfortunately, these solutions have inefficient thermal contact, resulting in reduced heat transfer, and may require 2–3 times longer (~7 minutes in a dry bead bath vs. ∼2.5 minutes in a 37°C waterbath for a standard cryovial) to thaw samples. This slower rate of thaw may be negatively impactful to the cell product.
Innovations in water-free automated thawing technology have enabled sample thawing with similar thawing rates as waterbaths (Figure 9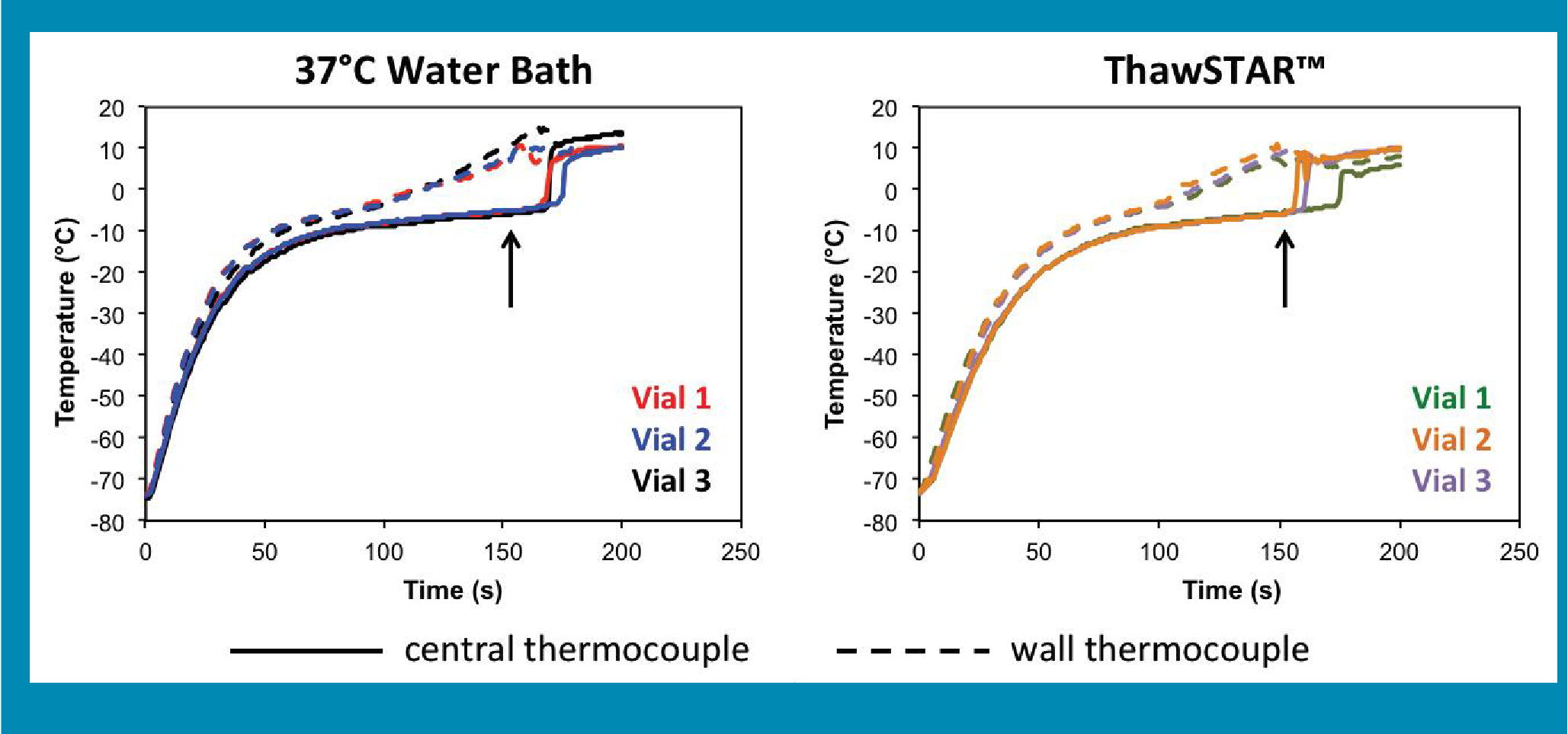
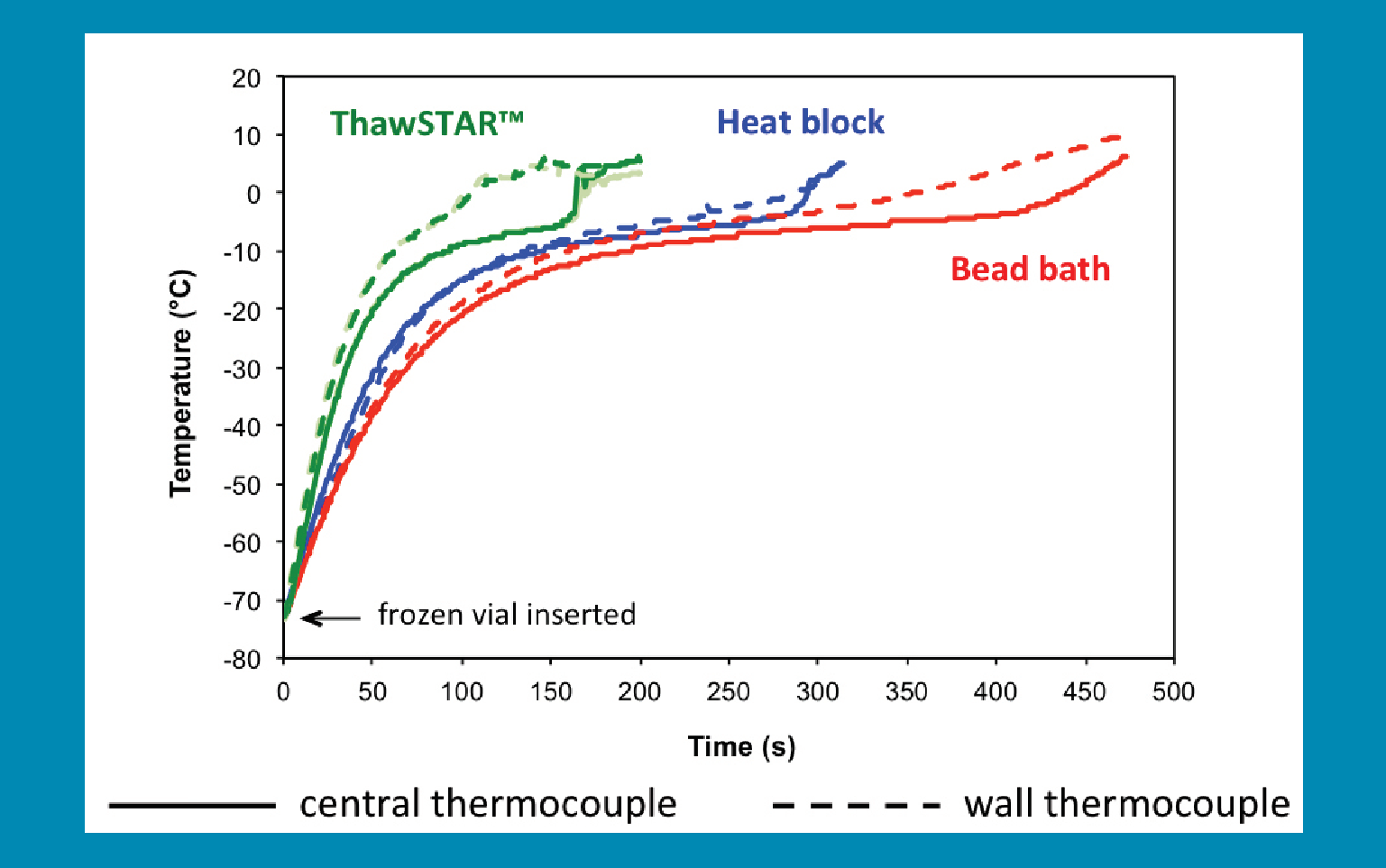
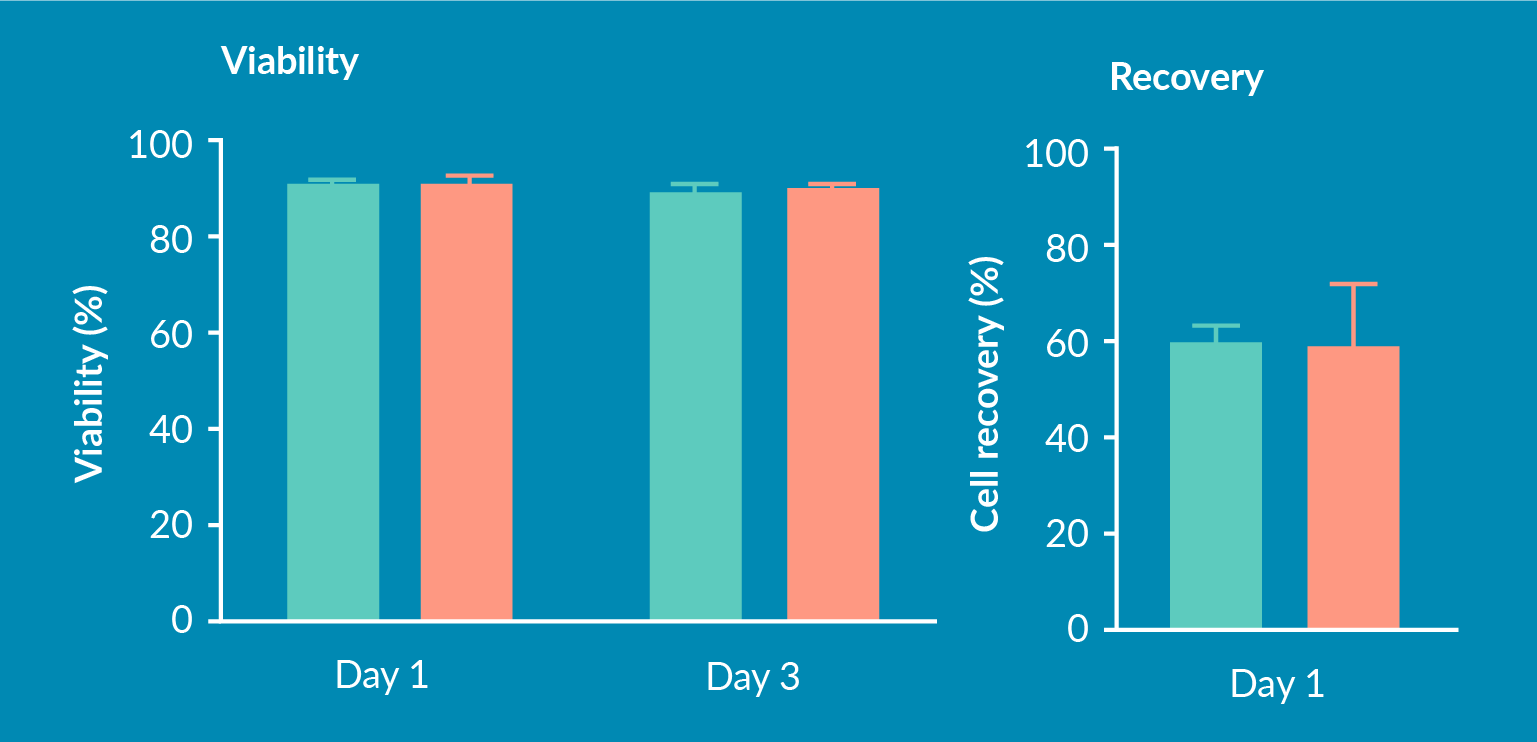
Conclusion
Cell and gene therapies are demonstrating clinical efficacy, and exhibiting early potential for commercial viability. The manufacturing and supply chain for cell and gene therapies would still benefit from substantial development and innovation, in order to model the robustness and efficiencies as experienced in the more mature fields of small molecule pharmaceuticals and large molecule biopharmaceuticals. Successful optimization of product development would benefit from a broad analysis of the product lifecycle and workflow. A methodical and diligent review of cell-based materials stability risk points (in essence, a Biopreservation Quality by Design, or BQbD), consideration to Biopreservation Critical Process Parameters (BCPP), and identification of Biopreservation Critical Quality Attributes (BCQA); would serve to identify stability gaps, increase system robustness, and optimize the overall CGT manufacturing and supply chain workflow. Optimizing the end-to-end Process utilizing Biopreservation Best Practices, and integrating the latest tools and technologies related to biopreservation media, controlled rate freezing and cryogenic storage, cold chain shipping management, and automated water-free thawing; would facilitate optimization of the CGT Product, and increase the probabilities for clinical and commercial success.
References
1. Alliance for Regenerative Medicine Annual Report & Sector Year in Review: 2019. Crossref
2. Bersenev A, Kili S. Management of ‘out of specification’ commercial autologous CAR-T cell products. Cell Gene Ther. Ins. 2018; 4(11): 1051–8. Crossref
3. Chen LN, Collins-Johnson N, Sapp N, Pickett A, West K, Stroncek DF, Panch SR. How do I structure logistic processes in preparation for outsourcing of cellular therapy manufacturing? Transfusion 2019; 59: 2506–18. Crossref
4. Juliano L, Eastwood G, Berard T, Mathew AJ. The Importance of Collection, Processing and Biopreservation Best Practices in Determining CAR-T Starting Material Quality. Cell Gene Ther. Ins. 2018; 4(4): 327–36. Crossref
5. Hawkins BJ, Abazari A, Mathew AJ. Biopreservation Best Practices for regenerative medicine GMP manufacturing & focus on optimized biopreservation media. Cell Gene Ther. Ins. 2017; 3(5): 345–58. Crossref
6. Abazari A, Hawkins BJ, Clarke DM, Mathew AJ. Biopreservation Best Practices: A Cornerstone in the Supply Chain of Cell-based Therapies – MSC Model Case Study. Cell Gene Ther. Ins. 2017; 3(10): 853–71. Crossref
7. Clarke D, Smith D. Managing starting material stability to maximize manufacturing flexibility and downstream efficiency. Cell Gene Ther. Ins. 2019; 5(2): 303–14. Crossref
8. Abazari A. Process development considerations for cryopreservation of cellular therapies. Cell Gene Ther. Ins. 2019; 5(9): 1151–67. Crossref
9. Abazari A. Implementation of Biopreservation Best Practices to address a critical component of cell and gene therapy manufacturing: https://insights.bio/cell-and-gene-therapy-insights/implementation-of-biopreservation-best-practices-to-address-a-critical-component-of-cell-and-gene-therapy-manufacturing Crossref
10. Mathew AJ. Biopreservation Considerations for Regenerative Medicine GMP Manufacturing; 2018: http://ebook.liebertpub.com/biolife-solutions/biopreservation-considerations-for-regenerative-medicine-gmp-manufacturing Crossref
11. Mazur P. The role of intracellular freezing in the death of cells cooled at supraoptimal rates. Cryobiology 1977; 14: 251–72. Crossref
12. Chen HH, Clarke DM, Gao D. Direct concentration measurements of the unfrozen portion of solutions under freezing. Cryobiology 2010; 61: 161–5. Crossref
13. Berz D, McCormack EM, Winer ES et al. Cryopreservation of Hematopoietic Stem Cells. Am. J. Hematol. 2007; 82(6): 463–72. Crossref
14. KYMRIAH Prescribing Information, including Boxed WARNING, and Medication Guide: https://www.novartis.us/sites/www.novartis.us/files/kymriah.pdf Crossref
15. USP <1044> Cryopreservation of Cells. Sept 27, 2018: https://www.usp.org/sites/default/files/usp/document/our-work/biologics/resources/gc-1044-cryopreservation-of-cells.pdf Crossref
16. YESCARTA Product Information – European Medicines Agency: https://www.ema.europa.eu/en/documents/product-information/yescarta-epar-product-information_en.pdf Crossref
17. YESCARTA Product Monograph – Gilead Canada: http://www.gilead.ca/application/files/2715/8646/6805/Yescarta_English_PM_e214145-GS-002-Clean.pdf Crossref
18. Zynteglo Product Information – European Medicines Agency: https://www.ema.europa.eu/en/documents/product-information/zynteglo-epar-product-information_en.pdf Crossref
19. TECARTUS Package Insert – US FDA: https://www.fda.gov/media/140409/download Crossref
20. Nicoud IA, Clarke DM, Taber G, Stolowski KM, Roberge SE, Song MK, Mathew AJ, Reems J. Cryopreservation of umbilical cord blood with a novel freezing solution that mimics intracellular ionic composition. Transfusion 2012; 52(9): 2055–62. Crossref
21. Lawson A, Mukherjee IN, Sambanis A. Mathematical modeling of cryoprotectant addition and removal for the cryopreservation of engineered or natural tissues. Cryobiology 2012; 64(1): 1–11. Crossref
22. Best BP. Cryoprotectant Toxicity: Facts, Issues, and Questions. Rejuvenation Res. 2015; 18(5): 422–36. Crossref
23. Morris GJ, Acton A. Controlled ice nucleation in cryopreservation – A review. Cryobiology 2013; 66: 85–92. Crossref
24. BioLife Solutions Cryopreservation Protocol: https://www.biolifesolutions.com/wp-content/uploads/2018/01/6012_07-CryoStor-Product-Information-Sheet.pdf Crossref
25. Creer MH, Mathew AJ, Lemas MV. Practical Handbook of Cellular Therapy Cryopreservation. AABB Press 2015. Crossref
26. Baboo J, Kilbride P, Delahaye M et al. The Impact of Varying Cooling and Thawing Rates on the Quality of Cryopreserved Human Peripheral Blood T Cells. Sci. Rep. 2019; 9: 3417. Crossref
27. Meneghel J, Kilbride P, Morris GJ et al. Physical events occurring during the cryopreservation of immortalized human T cells. PLoS ONE 2019; 14(5). Crossref
28. Kofanova OA, Davis K, Glazer B et al. Viable Mononuclear Cell Stability Study for Implementation in a Proficiency Testing Program: Impact of Shipment Conditions. Biopreserv. Biobank. 2014; 12(3): 206–16. Crossref
29. Abazari A, Hawkins BJ, Fink J, O’Donnell K, Mathew AJ. Next Generation Technology, Procedures, and Products Facilitate Biopreservation Best Practices for Cellular Therapies. 2016: https://www.biolifesolutions.com/wp-content/uploads/2016/10/Biolife_Brooks_Whitepaper_OCT20_REL.pdf Crossref
30. Awan M, Buriak I, Fleck R et al. Dimethyl sulfoxide: a central player since the dawn of cryobiology, is efficacy balanced by toxicity? Regen. Med. 2020; 15(3): 1463–91. Crossref
31. O’Donnell K. Moving from passive to rescue design packaging: helping cells arrive alive with smart shippers. Cell Gene Ther. Ins. 2015; 1(2): 163–71. Crossref
32. O’Donnell K, Mathew AJ. Cell and Gene Therapies in Transit: Caution – Hazards Ahead. Cell Gene Ther. Ins. 2016: https://www.insights.bio/cell-and-gene-therapy-insights/journal/article/474/Cell-and-Gene-Therapies-in-Transit-Caution-Hazards-Ahead. Crossref
33. United Airlines Cargo, SAVSU. Transforming the future of medical shipments. 2019: https://ual.unitedcargo.com/Transforming-the-future-of-medical-shipments-SOCIAL-Page. Crossref
34. Röllig C, Babatz J, Wagner I et al. Thawing of cryopreserved mobilized peripheral blood--comparison between waterbath and dry warming device. Cytotherapy 2002; 4(6): 551–5. Crossref
35. Triana E, Ortega S, Azqueta C et al. Thawing of cryopreserved hematopoietic progenitor cells from apheresis with a new dry-warming device. Transfusion 2013; 53(1): 85–90. Crossref
36. ThawSTAR Automated Cell Thawing System: https://www.biolifesolutions.com/wp-content/uploads/2020/02/BioLife-ThawSTAR-Catalog.pdf Crossref
Affiliations
Todd CJ Berard
BioLife Solutions Inc.
Aby J Mathew
BioLife Solutions Inc.
Authorship & Conflict of Interest
Contributions: All named authors take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Acknowledgements: None.
Disclosure and potential conflicts of interest: Todd CJ Berard and Aby J Mathew are employees of BioLife Solutions, Inc. Todd CJ Berard is Chief Marketing Officer. Aby J Mathew is Executive Vice President & Chief Scientific Officer.
Funding declaration: The authors received no financial support for the research, authorship and/or publication of this article.
Article & copyright information
Copyright: Published by Cell and Gene Therapy Insights under Creative Commons License Deed CC BY NC ND 4.0 which allows anyone to copy, distribute, and transmit the article provided it is properly attributed in the manner specified below. No commercial use without permission.
Attribution: Copyright © 2020 Biolife Solutions, Inc. Published by Cell and Gene Therapy Insights under Creative Commons License Deed CC BY NC ND 4.0.
Article source: Invited.
Submitted for peer review: Aug 19 2020; Revised manuscript received: Oct 20 2020; Publication date: Oct 29 2020.
