Design and production of antibody PEG-conjugates for extended ocular retention
Bioconjugation Insights 2025; 1(4), 143–156
DOI: 10.18609/bci.2025.026
Herein, we report the site-specific conjugation of polyethylene glycol polymers to increase the vitreal half-life of two antibodies that target a transmembrane receptor protein found in the eye. The resulting conjugates’ design, optimization, purification, and characterization are described. Surface plasmon resonance (SPR) analysis demonstrated that the PEG-conjugated antibodies retained binding affinity to the target proteins, compared to the parental unconjugated antibody. The PEG-conjugated antibodies exhibited a slower rate of vitreal clearance compared to the unmodified antibodies in a New Zealand white rabbit ocular pharmacokinetic study.
Protein therapeutics developed for treating ocular diseases require dosing as frequently as once per month due to their short vitreal half-lives [1]Ferreira A, Sagkriotis A, Olson M, Lu J, Makin C, Milnes F. Treatment frequency and dosing interval of ranibizumab and aflibercept for neovascular age-related macular degeneration in routine clinical practice in the USA. PLoS ONE 2015; 10, e0133968. . A slower rate of vitreal clearance can reduce dosing frequency, likely improving patient compliance and positively affecting treatment outcomes [2]Ehlken C, Helms M, Böhringer D, Agostini HT, Stahl A. Association of treatment adherence with real-life VA outcomes in AMD, DME, and BRVO patients. Clin. Ophthalmol. 2018; 12, 13–20. . Increasing the apparent hydrodynamic radius of protein therapeutics has been shown to slow the rate of vitreal clearance [3]Shatz W, Hass PE, Mathieu M, et al. Contribution of antibody hydrodynamic size to vitreal clearance revealed through rabbit studies using a species-matched fab. Mol. Pharm. 2016; 13, 2996–3003. . Modifying antibodies, their fragments or mimetics with polymers, such as polyethylene glycol (PEG) [3]Shatz W, Hass PE, Mathieu M, et al. Contribution of antibody hydrodynamic size to vitreal clearance revealed through rabbit studies using a species-matched fab. Mol. Pharm. 2016; 13, 2996–3003. [4]Shatz W, Hass PE, Peer N, et al. Identification and characterization of an octameric PEG-protein conjugate system for intravitreal long-acting delivery to the back of the eye. PLoS ONE 2018; 14, e0218613. [5]Sharma A, Kumar N, Kuppermann BD, Bandello F. Abicipar pegol: the non-monoclonal antibody anti-VEGF. Eye 2020; 34, 797–801. [6]Vollmar BS, Fei M, Liang WC, et al. PEGylation of anti-MerTK antibody modulates ocular biodistribution. Bioconjug. Chem. 2022; 33, 1837–1851. [7]Famili A, Crowell SR, Loyet KM, et al. Hyaluronic acid-antibody fragment bioconjugates for extended ocular pharmacokinetics. Bioconjug. Chem. 2019; 30, 2782–2789. , or phosphorylcholine biopolymers [8]Chandrasekaran PR, Madanagopalan VG. KSI-301: antibody biopolymer conjugate in retinal disorders. Ther. Adv. Ophthalmol. 2021; 13. increases the hydrodynamic radius and thus the vitreal half-lives. To attach a polymer of interest to an antibody therapeutic, the conjugation strategy should be site-specific, proceed with high conversion, and minimally affect antibody function [9]Fisusi F, Brandy N, Wu J, Akala EO. Studies on polyethylene glycol-monoclonal antibody conjugates for fabrication of nanoparticles for biomedical applications. J. Nanosci. Nanomed. 2020; 4, 1–9. [10]Rodwell J, McKearn T. Linker technology: antibody-mediated delivery systems. Nat. Biotechnol. 1985; 3, 889–894..
Herein, the design and chemical conjugation of PEG polymers to an antibody and an antibody-receptor fusion that target a transmembrane receptor protein are described. The resulting conjugates are assessed for purity, in vitro binding, and in vivo ocular retention in a New Zealand white rabbit pharmacokinetic study.
Materials & methods
Materials
Cysteamine hydrochloride and Tris (2-carboxyethyl) phosphine hydrochloride (TCEP-HCl) were purchased from Sigma-Aldrich (St Louis, MO, USA). Methoxy-PEG-(CH2)3NHCO(CH2)2-MAL MW 5,000 (α-[3-(3-Maleimido-1-oxopropyl)amino]propyl-ω-methoxy, polyoxyethylene), Methoxy-PEG-(CH2)3NHCO(CH2)2-MAL, MW 20,000 (α-[3-(3-Maleimido-1-oxopropyl)amino]propyl-ω-methoxy, polyoxyethylene), and 2 arm branched PEG,-(CH2)3NHCO(CH2)2-MAL, MW 40,000 (2,3-Bis(methylpolyoxyethylene-oxy)-1-{[3-(3-maleimido-1-oxopropyl)amino]propyloxy} propane) were obtained from NOF America Corporation (White Plains, NY). According to the manufacturer, the Polydispersity Index (PDI) for all polymers was ~1.1. Endotoxin levels were measured using the Endosafe® Nexgen-PTS instrument using 0.5–0.005 EU/mL CRL test cartridges sourced from Charles River Laboratories (Wilmington, MA, USA).
Protein expression, bioconjugation, and purification
Antibodies were expressed using the ThermoFisher Scientific (Waltham, MA) Expi293™ transient expression system according to the manufacturer’s protocols. Clarified cell culture supernatant was purified with a HiTrap MabSelect PrismA™ column (Cytiva, Marlborough, MA), equilibrating with 20 mM sodium phosphate, 150 mM NaCl, pH 7.2. Antibodies captured on the column were eluted with 100 mM sodium citrate, pH 3.5, and neutralized with 1M Tris, pH 8.0 (Teknova, Hollister, CA, USA).
Following purification, antibodies were exchanged into 50 mM sodium phosphate, 150 mM NaCl, 2.5 mM EDTA, pH 7.2. Antibodies at 20 µM were reduced with either cysteamine-HCl (50 mM) for 1 h or TCEP-HCl (1 mM) for 15 min at room temperature. Following reduction, excess reducing agent was removed using a HiTrap™ Desalting column (Cytiva) equilibrated with 50mM sodium phosphate, 15 mM NaCl, 2.5 mM EDTA, pH 7.2. Following desalting, a 10-fold molar excess of either 5 kDa PEG, 20 kDa PEG, or 40 kDa PEG was added to the reduced material, and the reaction proceeded at room temperature for 1.5 h. PEGylated material was then captured on a HiTrap SP High Performance column (Cytiva) equilibrated with 20 mM sodium acetate, pH 4.6, and eluted over a linear gradient with 20 mM sodium acetate, 1 M NaCl, pH 4.6.
Characterization
SDS-PAGE
NuPAGE™ 3–8% tris-acetate or NuPAGE 4–12% bis-tris gels (ThermoFisher Scientific) were used for SDS-PAGE analysis. Color-coded pre-stained protein marker 10–250 kDa from Cell Signaling Technology (Danvers, MA, USA) was used as a molecular weight marker. Non-reduced protein samples (5 µg) were combined with 4x lithium dodecyl sulfate (LDS), and the samples were run without heating. Reduced protein samples (5 µg) were combined with dithiothreitol (5 mM) in 4× LDS and heated for 5 minutes at 95 °C. Gels were stained with SimplyBlue™ SafeStain (ThermoFisher Scientific) according to the manufacturer’s instructions.
HPLC
High-pressure liquid chromatography (HPLC) was performed on an Agilent (Santa Clara, CA, USA) 1100 Series HPLC at room temperature. Sample analysis for all HPLC experiments was achieved with an inline diode array detector (DAD) and monitoring absorbance at 280 nm (A280). Chromatograms were analyzed using the Agilent ChemStation software. For Size Exclusion High Pressure Liquid Chromatography (SEC-HPLC), protein samples (10 µg) were injected into a Sepax Technologies (Newark, DE) Zenix SEC-300, 3 µm, 300 Å, 7.8 x 300 mm column flowing at 0.5 mL/min for 40 min. The mobile phase was 1X PBS, 100 mM arginine, 0.5 mM EDTA, pH 6.7. A Bio-Rad (Hercules, CA, USA) gel filtration standard was used to relate the retention time to the expected molecular weight. For Reversed Phase Liquid Chromatography (RP-HPLC), protein samples (10 µg) were injected onto an Agilent AdvanceBio RP-mAb Diphenyl, 4.6 × 150 mm, 3.5 µm, flowing at 0.5 mL/min. The mobile phase was a linear gradient of 0.1% trifluoroacetic acid (TFA) in HPLC-grade water and 0.1% TFA in HPLC-grade acetonitrile sourced from ThermoFisher Scientific (Waltham, MA, USA).
SPR
Surface plasmon resonance (SPR) experiments were performed on a Biacore3000 instrument using CM5 sensor chips (Cytiva) at 25 °C. Antigen immobilization was carried out using N-hydroxysuccinimide (NHS)/1-Ethyl-3-[3-dimethylaminopropyl]carbodiimide (EDC) coupling. A 100 nM, 33.3 nM, 11.1 nM, 3.7 nM, and 1.2 nM solution of each antibody was injected at 30 µL/min for 300 s over a surface containing immobilized antigen, and dissociation was monitored for 900 s. The combined kinetic traces were fit to a 1:1 interaction model using Scrubber software (BioLogic Software, Canberra, Australia), and the equilibrium dissociation constant (KD) and standard deviation are based on the combined fit in a single experiment.
Ocular pharmacokinetic (PK) study
Formulation
EYLEA® was purchased from Myonex (Horsham, PA) and diluted into the EYLEA formulation buffer (10 mM sodium phosphate, 40 mM NaCl, 0.03% polysorbate-20, 5% sucrose) such that the final aflibercept concentration was 2 mg/mL. All other test articles were formulated in 1X PBST (phosphate-buffered saline containing 0.01% polysorbate-20) at 2 mg/mL and 10 mg/mL for the PRO085 and PRO504 ocular PK studies, respectively. All test articles were filtered through a 0.22 µm Steriflip filter (Millipore, Burlington, MA, USA), and endotoxin was measured below 0.1 EU/mg.
Study design
For the PK study on PRO085 and PEGylated variants, 100 µg intravitreal injections were administered to male New Zealand White rabbits. For each cohort, six animals were dosed on day zero. On days 1, 3, and 7, animals were euthanized, and both eyes were harvested and frozen prior to dissection and collection of vitreous humor. The vitreous humor samples were diluted 1:5 in 1X PBST (phosphate-buffered saline with 0.1% polysorbate-20) without homogenization and stored at −70 °C until analysis.
For the PK study on PRO504 and PEGylated variants, 500 µg intravitreal injections were administered to male New Zealand White rabbits. For each cohort, three or four animals were dosed on day 0. On days 1, 3, 7, and 14, animals were euthanized, and both eyes were harvested and frozen prior to dissection and collection of vitreous humor. The vitreous humor samples were diluted 1:5 in 1× PBST (phosphate-buffered saline with 0.1% polysorbate-20) without homogenization and stored at −70 °C until analysis.
Enzyme-linked immunosorbent assay (ELISA)
Total drug levels in the vitreous humor were quantified using two different ELISAs. All incubations were performed with shaking at 420 rpm unless otherwise indicated. High-binding microtiter plates (Greiner Bio One, Monroe, NC, USA) were coated overnight at 4 °C with an anti-human IgG in 100 mM carbonate, pH 9.5, without agitation. The coated plate was washed with 1× PBST (phosphate-buffered saline with 0.1% polysorbate-20) containing 150 mM sodium chloride and blocked with 5% bovine serum albumin in 1X PBST for 1 h at 37 °C. After washing, test articles and their corresponding controls were incubated for 1.5 h at room temperature.
For PRO085, PRO171 + [2 × 5 kDa PEG], PRO171 + [2 × 20 kDa PEG], PRO504, PRO593 + [2 × 20 kDa PEG], the plates were washed and incubated with biotinylated antigen for 1.5 h at room temperature. The plates were rewashed, incubated with streptavidin conjugated HRP (R&D Systems, Minneapolis, MN) for 1 h at room temperature, and protected from light.
For aflibercept, the plates were washed and then incubated with a polyclonal goat anti-human IgG-HRP conjugate for 1 h at room temperature and protected from light.
The plate was washed a final time, developed with WesternBright chemiluminescent HRP substrate (Advansta Inc., San Jose, CA, USA) according to the manufacturer’s protocols, and read in a Molecular Devices SpectraMax® M5 plate reader.
Results & discussion
Antibodies PRO085 and PRO504, depicted in the illustrations in Figure 1, were selected for this study. These antibodies target the extracellular domain of a one-pass transmembrane protein receptor (protein A, target cannot be disclosed), and PRO504 contains a C-terminal fusion that binds to a soluble signaling protein (protein B, target cannot be disclosed). To achieve site-specific cysteine modification with maleimide-functionalized polyethylene glycol (PEG), several variants of the parent antibodies were produced, and the conjugation conditions were optimized for each construct. For each parent antibody, one cysteine conjugation variant was selected to be scaled and evaluated in a rabbit ocular pharmacokinetic study.
| Figure 1. The target parent antibodies, PRO085 and PRO504. |
|---|
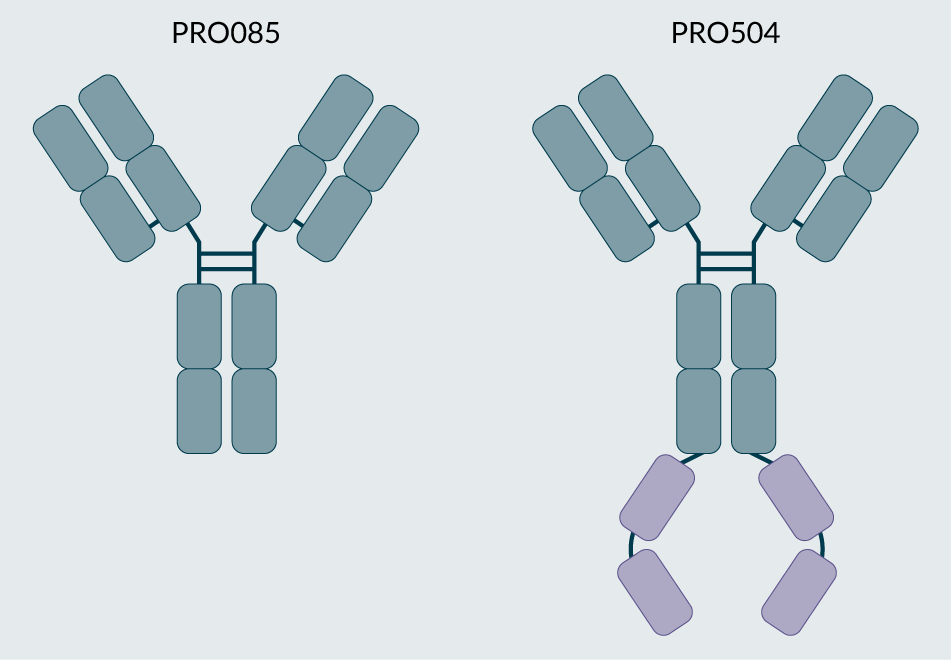 |
| © 2025, BioInsights Publishing Ltd. All rights reserved. |
PRO085 construct design
Several N-terminal and C-terminal PRO085 variants were designed and produced, as shown in Figure 2. To target the N-terminus of the light chain, an unpaired cysteine residue was directly appended to the parental light chain (PRO086) or included in a five-residue N-terminal linker (DICGS, PRO087, or CDGSG, PRO088). Analogous constructs were made to target the N-terminus of the heavy chain, including an appended cysteine (PRO140) and five-residue linkers (PRO141 and PRO142). To target the C-terminus of the light chain, nine residue extensions comprised of glycine-serine flexible spacers were chosen to flank the unpaired cysteine residue (PRO144). For PRO143, a lysine residue was positioned next to the unpaired cysteine residue to lower the pKa of the reactive cysteine, thus improving its ability to be modified site-selectively [11]Jao SC, Ospina SME, Berdis AJ, Starke DW, Post CB, Mieyal JJ. Computational and mutational analysis of human glutaredoxin (thioltransferase): probing the molecular basis of the low pKa of cysteine 22 and its role in catalysis. Biochemistry 2006; 45, 4785–4796. . A similar approach was selected to target the C-terminus of the heavy chain, as shown in Figure 2D for PRO145. Several highly reactive engineered cysteine residues have been described, and we sought to apply this strategy to the conjugation of PEG to PRO085 by introducing the reported V205C (PRO172), S112C (PRO173), A114C (PRO174), and T116C (PRO175) mutations [12]Junutula JR, Raab H, Clark S, et al. Site-specific conjugation of a cytotoxic drug to an antibody improves the therapeutic index. Nat. Biotechnol. 2008; 26, 925–932. . Finally, we introduced heavy chain C218S, C227S, and light chain C214S point mutations to target the modification of the upper hinge, Cys 224 [13]Kang JC, Sun W, Khare P, et al. Engineering a HER2-specific antibody–drug conjugate to increase lysosomal delivery and therapeutic efficacy. Nat. Biotechnol. 2019; 37, 523–526. .
| Figure 2. Schematics for all PRO085 variants for cysteine PEGylation. |
|---|
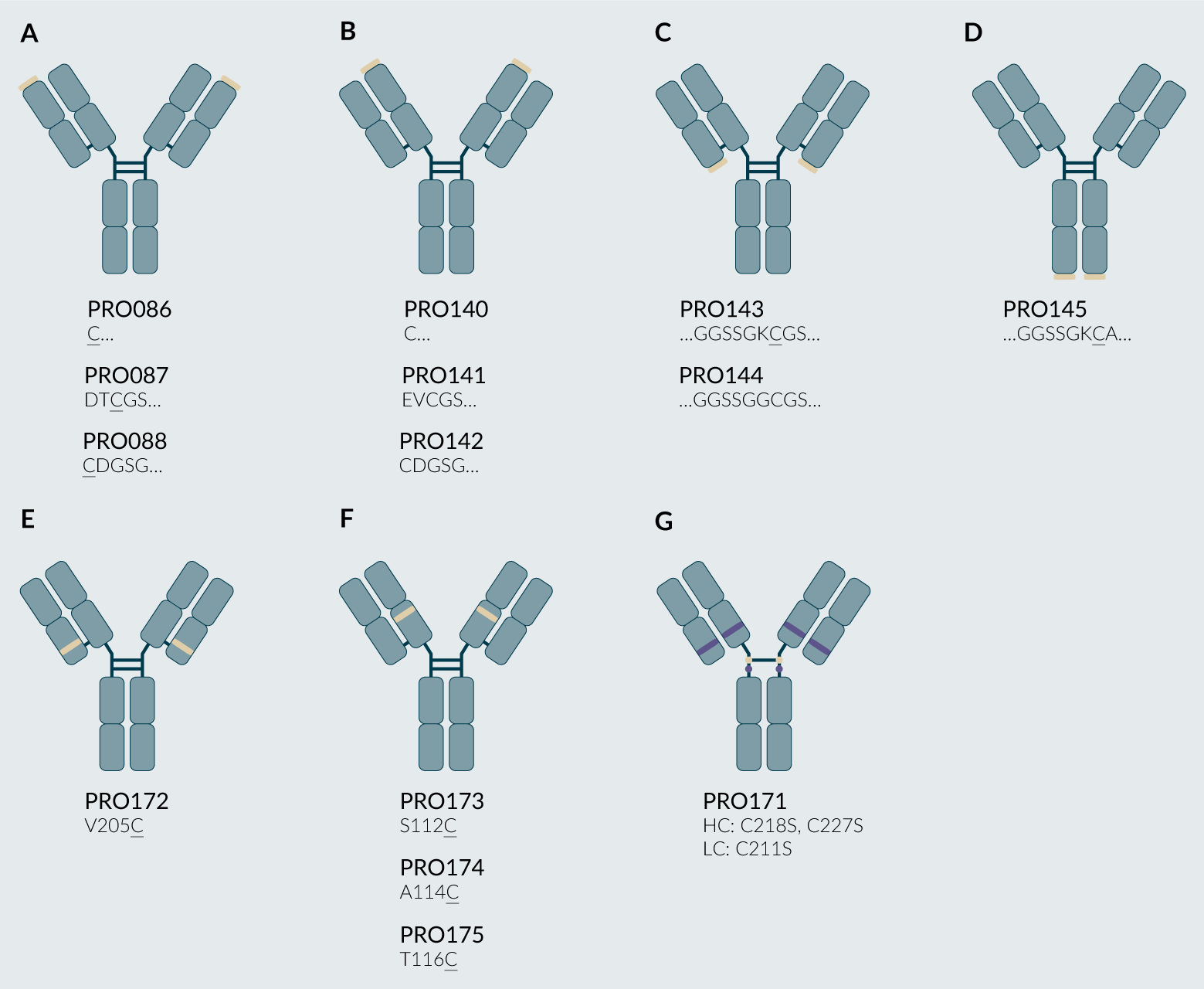 |
(A) N-terminal light chain variants (PRO086, PRO087, PRO088). (B) N-terminal heavy chain variants (PRO140, PRO141, PRO142). (C) C-terminal light chain variants (PRO143, PRO144). (D) C-terminal heavy chain variants (PRO145). (E) light chain variants (PRO172). (F) heavy chain variants (PRO173, PRO174, PRO175). (G) heavy chain C218S, C227S and light chain C214S to modify the heavy chain C224. Light brown ovals represent targeted cysteine residues (native or mutant), and purple ovals represent residues mutated from cysteine to serine. © 2025, BioInsights Publishing Ltd. All rights reserved. |
PRO085 PEG conjugation
Following reported reduction, re-oxidation, and conjugation procedures, PRO086-PRO088, PRO140-PRO145, and PRO171-PRO175 were evaluated for modification with maleimide-functionalized 5 kDa or 20 kDa linear PEG [12]Junutula JR, Raab H, Clark S, et al. Site-specific conjugation of a cytotoxic drug to an antibody improves the therapeutic index. Nat. Biotechnol. 2008; 26, 925–932. .
N-terminal cysteines were found to be more readily modified than C-terminal cysteines, and cysteines on the heavy chain were more readily modified than those on the light chain ([PRO140, PRO141, PRO142] > [PRO086, PRO087, PRO088] > PRO145 > [PRO143, PRO144]). All antibodies were assessed for binding to their intended target, A and B, using SPR, where the primary objective was to verify the interaction between the PEG-conjugated compounds. Including a mixture of vitreous proteins could introduce non-specific binding, thereby complicating the interpretation of the reported dissociation constants (KDs) for the targets of interest. Antibodies conjugated at N-terminal cysteines showed decreased binding signal via SPR to Target A relative to those modified at C-terminal cysteines.
Modification occurred more readily on light chain V205C (PRO172) and heavy chain A114C (PRO174) than heavy chain S112C (PRO173) and heavy chain T116C (PRO175).
Upon optimization of reduction and conjugation conditions, PRO171 converted into ~65% of the desired [2 × PEG] conjugated antibody, as determined by SDS-PAGE (schematic shown in Figure 3). Due to the high conversion to the desired doubly PEG modified product, PRO171 was selected to scale and evaluate in a rabbit ocular pharmacokinetic study.
| Figure 3. Optimized reduction and conjugation strategy for PRO171 to yield PRO171 + [2 × 5 kDa PEG] or PRO171 + [2 × 20 kDa PEG]. |
|---|
![Optimized reduction and conjugation strategy for PRO171 to yield PRO171 + [2 × 5 kDa PEG] or PRO171 + [2 × 20 kDa PEG].](https://cdn.insights.bio/uploads/BCI Mobs F3 132.png) |
| © 2025, BioInsights Publishing Ltd. All rights reserved. |
Following the scaled 5 kDa and 20 kDa PEG conjugation of PRO171, the material was purified using a strong cation exchange column on a fast protein liquid chromatography (FPLC) system, and the resulting fractions were assessed for purity by SDS-PAGE, as shown in Figure 4A and B. PRO171 + [2 × PEG] eluted early in the gradient, followed by the PRO171 + [1 × PEG], and unmodified PRO171. Fractions corresponding to doubly PEG-modified PRO171 were pooled and analyzed by SDS-PAGE, SEC-HPLC, RP-HPLC, and binding by SPR, as shown in Figure 5A–D.
| Figure 4. Purification of PRO171 + [2 × 5 kDa PEG] and PRO171 + [2 × 20 kDa PEG]. |
|---|
![Purification of PRO171 + [2 × 5 kDa PEG] and PRO171 + [2 × 20 kDa PEG].](https://cdn.insights.bio/uploads/BCI Mobs F4 154.png) |
| (A) Cation exchange chromatography was used to separate PRO171 + [2 × 5 kDa PEG] and PRO171 + [2 × 20 kDa PEG] from unmodified or singly modified PRO171. Representative fast protein liquid chromatography (FPLC) traces. (B) Reduced SDS-PAGE are shown above for PRO171 + [2 × 5 kDa PEG] and PRO171 + [2 x 20 kDa PEG], highlighted in blue and green, respectively. For the SDS-PAGE, the crude reaction mixture was run in lane 1, and all pooled fractions are outlined in the grey dotted box. |
| Figure 5. Characterization of PRO085, PRO171 + [2 × 5 kDa PEG], and PRO171 + [2 × 20 kDa PEG] by (A) SDS-PAGE, (B) SEC-HPLC, (C) RP-HPLC, and (D) SPR. |
|---|
![Characterization of PRO085, PRO171 + [2 × 5 kDa PEG], and PRO171 + [2 × 20 kDa PEG] by (A) SDS-PAGE, (B) SEC-HPLC, (C) RP-HPLC, and (D) SPR.](https://cdn.insights.bio/uploads/BCI Mobs F5 132.png) |
Non-reduced SDS-PAGE analysis revealed PRO085 migrates as a single band at the expected molecular weight. PRO171 + [2 × 5 kDa PEG] and PRO171 + [2 × 20 kDa PEG] have three separate bands when analyzed by SDS-PAGE. PRO171 lacks four disulfide bonds compared to PRO085, which likely influences its migration on SDS-PAGE. The top, middle, and bottom bands are tentatively assigned to the PEG conjugated heavy chain dimer, PEG conjugated heavy chain monomer, and unpaired light chain, respectively.
Analysis by SEC-HPLC measured at >90% main peak. PRO085, PRO171 + [2 × 5 kDa PEG], PRO171 + [2 × 20 kDa PEG] had retention times of 15.6 min, 14.4 min, and 12.3 min, respectively. The retention time for PRO085 is consistent with a typical retention time for antibodies, indicating that the antibody remains intact under native conditions. The comparison of retention times between unmodified and PEG-conjugated material indicates that PRO171 + [2 × 20 kDa PEG] has the largest hydrodynamic radius, followed by PRO171 + [2 × 5 kDa PEG], and finally PRO085, as expected.
By RP-HPLC, purities were >90% main peak. PRO171 + [2 × 20 kDa PEG] was retained the longest, followed by PRO171 + [2 × 5 kDa PEG], and finally PRO085. This retention time increases PEG length, with larger PEG species interacting more strongly with the stationary phase and being retained longer on the column, as expected.
PRO085, PRO171 + [2 × 5 kDa], and PRO171 + [2 × 20 kDa] PEG bound the soluble extracellular domain of the target receptor with comparable equilibrium binding constants (KD) of 0.81 nM, 0.76 nM, and 1.22 nM, respectively.
The stability of PEG-conjugated PRO085 was evaluated at 40 °C over 30 days. The primary degradation pathway involved the loss of one PEG moiety from the doubly modified PRO085, which plateaued at approximately 35%. Loss of both PEGs accounted for no more than 8% of the total protein. Despite this degradation, the material was deemed suitable for use in the ocular pharmacokinetic (PK) study described herein. Given the 7-day duration of the PK study, it is likely that the doubly PEG-conjugated species remained as the predominant form throughout the study.
PRO085 Ocular PK Study
Four cohorts of New Zealand White rabbits were dosed with 100 µg of PRO085, PRO171 + [2 × 5 kDa PEG], PRO171 + [2 × 20 kDa PEG], and aflibercept, respectively. An ELISA was used to analyze vitreous humor samples collected on days 1, 3, and 7 for total drug, and the half-life was determined for the values fit to a log-linear regression. As shown in Figure 6A, the half-life was 3.4 days for aflibercept, similar to the reported value of 3.9 days [14]Park SJ, Choi Y, Na YM, et al. Intraocular pharmacokinetics of intravitreal aflibercept (Eylea) in a rabbit model. Invest. Ophthalmol. Vis. Sci. 2016; 57(6), 2612–2617. . PRO171 + [2 × 20 kDa PEG] was retained the longest in the vitreous with a measured half-life of 4.8 days, followed by PRO171 + [2 × 5 kDa PEG] at 3.5 days, and finally unmodified PRO085 at 2.9 days, as shown in Figure 6B–D.
| Figure 6. Total drug measured in vitreous humor by ELISA for (A) aflibercept, (B) PRO085, (C) PRO171 + [2 × 5 kDa PEG], and (D) PRO171 + [2 × 20 kDa PEG]. |
|---|
![Total drug measured in vitreous humor by ELISA for (A) aflibercept, (B) PRO085, (C) PRO171 + [2 × 5 kDa PEG], and (D) PRO171 + [2 × 20 kDa PEG].](https://cdn.insights.bio/uploads/BCI Mobs F6 132.png) |
PRO504 construct design
Guided by insights from the work with PRO085, a focused set of constructs was designed to enable efficient PEG conjugation (Figure 7). All variants included the light chain C214S mutation that reduces undesired light chain PEG conjugation of the PRO085 variants described above. PRO592 included the heavy chain C227S mutation to target modification of the upper hinge C224, while PRO596 included heavy chain C224S mutation to target modification of the lower hinge, C227. PRO593, PRO594, and PRO595 included mutations of C224S and C227S to remove the heavy chain hinge cysteines, thus preventing undesired PEGylation sites. In addition, unpaired cysteines were introduced on either the C-terminus (PRO593) or directly before (PRO594) or after (PRO595) a linker connecting the C-terminal fusion protein.
| Figure 7. All PRO504 variants for cysteine PEGylation. |
|---|
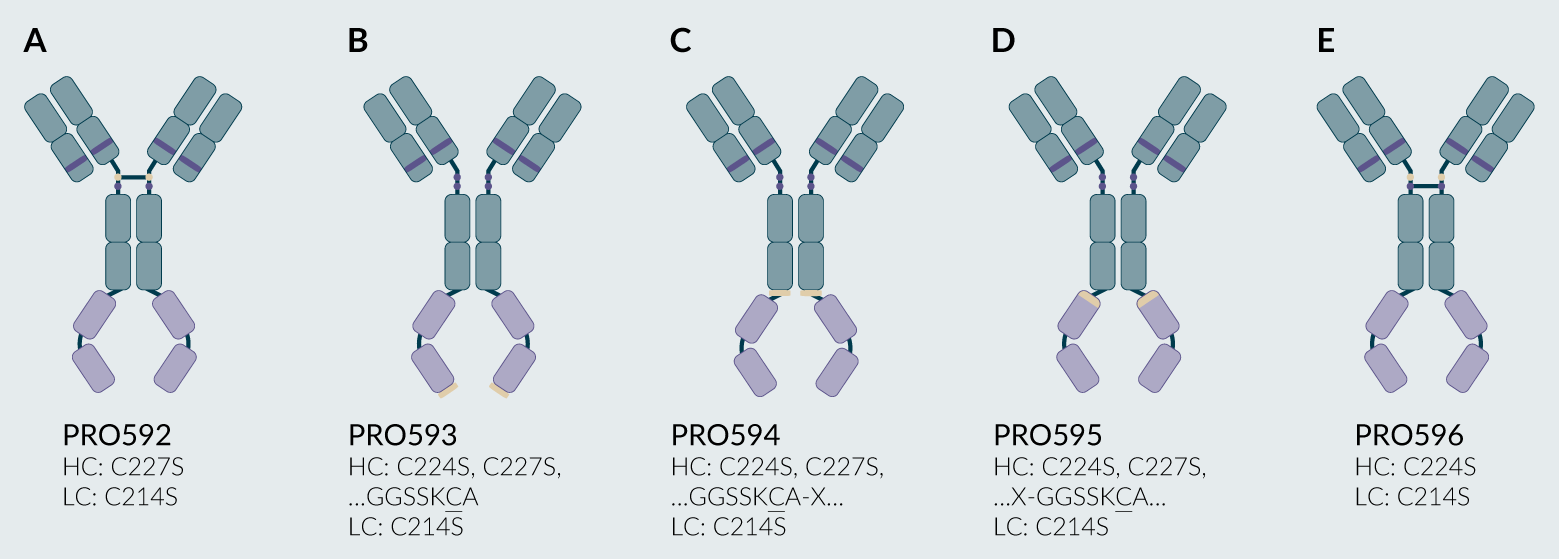 |
| All variants included the light chain C214S mutation as well as heavy chain mutations including: (A) C227S (PRO592); (B) C224S, C227S and a C-terminal extension (PRO593); (C) C224S and C227S with an introduced interior cysteine (PRO594); (D) C224S and C227S with an introduced interior cysteine (PRO595); and (E) C224S (PRO596). Light brown ovals represent targeted cysteine residues (native or mutant), and purple ovals represent residues mutated from cysteine to serine. For PRO594 and PRO595, the ‘X’ denotes an undisclosed linker. © 2025, BioInsights Publishing Ltd. All rights reserved. |
Upon optimization of reduction and conjugation conditions, PRO592 and PRO596 converted to 75% and 40% of the desired doubly PEG conjugated product, as assessed by SDS-PAGE. All constructs lacking the hinge disulfide bonds (PRO593, PRO594, PRO595) had near quantitative conversion to the desired doubly PEG conjugated product as assessed by SDS-PAGE. C-terminal variant PRO593 was selected for scaled conjugation and subsequent evaluation in a rabbit ocular pharmacokinetic study due to its high conversion to the desired product and reduced impact of conjugation on stability and target protein binding.
A 40 kDa branched PEG was selected for scaled conjugation to PRO593 to further extend the ocular retention compared to 5 kDa or 20 kDa PEG conjugates (Figure 8). Following the scaled 40 kDa PEG conjugation of PRO593, the material was purified using a strong cation exchange column on an FPLC system, and the resulting fractions were assessed for purity by SDS-PAGE. PRO593 + [2 × 40 kDa PEG] was found to elute early in the gradient, followed by unmodified PRO593. Fractions corresponding to doubly PEG-modified PRO593 were pooled and analyzed by SDS-PAGE, SEC-HPLC, and binding by SPR, as shown in Figure 9.
| Figure 8. Optimized reduction and conjugation strategy for PRO593 to yield PRO593 + [2 × 40 kDa PEG]. |
|---|
![Optimized reduction and conjugation strategy for PRO593 to yield PRO593 + [2 × 40 kDa PEG].](https://cdn.insights.bio/uploads/BCI Mobs F8 132.png) |
| © 2025, BioInsights Publishing Ltd. All rights reserved. |
| Figure 9. Characterization of PRO504 and PRO593 + [2 × 40 kDa PEG] by (A) SDS-PAGE, (B) SEC-HPLC, and (C) KD to target proteins A and B by SPR. |
|---|
![Characterization of PRO504 and PRO593 + [2 × 40 kDa PEG] by (A) SDS-PAGE, (B) SEC-HPLC, and (C) KD to target proteins A and B by SPR.](https://cdn.insights.bio/uploads/BCI Mobs F9 132.png) |
Non-reduced SDS-PAGE analysis revealed PRO504 migrates as a single band at the expected molecular weight and purity of >90% by densitometry, whereas PRO593 + [2 × 40 kDa PEG] migrates as four bands. PRO593 lacks four disulfide bonds compared to PRO504, which likely allows for dissociation under the denaturing conditions of the gel electrophoresis. From slowest to fastest migration, the bands are assigned as high molecular weight species (~8%), the PEG conjugated heavy chain dimer (~55%), the PEG conjugated heavy chain monomer (~6%), and the unpaired light chain (~31%).
Analysis by SEC-HPLC measured at >90% main peak, indicating that the dissociation observed in the electrophoresis does not occur under non-denaturing conditions. PRO504 and PRO593 + [2 × 40 kDa PEG] had retention times of 13.5 min and 11.1 min. The comparison of retention times between unmodified and PEG-conjugated material indicates that PRO593 + [2 × 40 kDa PEG] has a larger hydrodynamic radius than PRO504, as expected.
Unmodified PRO504 and PRO593 + [2 × 40 kDa PEG] bound the extracellular domain of the target receptor, protein A, and the soluble-signaling protein B, with comparable equilibrium binding constants (Figure 9C).
After confirming binding and purity, the test articles were formulated, tested for endotoxin, and used in an ocular pharmacokinetics study.
PRO504 Ocular PK Study
The half-lives of PRO504 and PRO593 + [2 × 40 kDa PEG] in the vitreous of New Zealand rabbits were 4.8 and 8 days, respectively (Figure 10A and B). As expected, the PEG-conjugated construct was retained longer in the vitreous due to its larger hydrodynamic radius.
| Figure 10. Total drug measured in vitreous humor by ELISA for (A) PRO504, and (B) PRO593 + [2 × 40 kDa PEG]. |
|---|
![Total drug measured in vitreous humor by ELISA for (A) PRO504, and (B) PRO593 + [2 × 40 kDa PEG].](https://cdn.insights.bio/uploads/BCI Mobs F10 132.png) |
Conclusions
Cysteine variants were designed and evaluated for an antibody and an antibody-receptor domain fusion protein, PRO085 and PRO504, to enable the attachment of a maleimide-functionalized PEG and extend the ocular retention of each protein. After an initial screen of 14 variants for PRO085, PRO171 was selected for scale-up due to its efficient conversion to the desired doubly PEG-conjugated antibody. PRO171 + [2 × 5 kDa PEG] and PRO171 + [2 × 20 kDa PEG] were >90% pure and bound the target antigen comparably to the parental antibody, PRO085. In an ocular pharmacokinetic study, PRO171 + [2 × 20 kDa PEG] had the most extended vitreous half-life of 4.8 days, followed by PRO171 + [2 × 5 kDa PEG] at 3.5 days, and finally unmodified PRO085 at 2.9 days. The half-life of PRO17 + [2 × 20 kDa PEG] was also superior to the approved intravitreal drug aflibercept.
Applying the insights from PRO085 conjugation efforts, 5 cysteine variants were produced for PRO504, and PRO593 was selected for scale-up due to its near quantitative conversion to the desired doubly PEG conjugated product. PRO593 + [2 × 40 kDa PEG] was >90% main peak and bound the two target proteins comparably to the parental PRO504. In an ocular pharmacokinetic study, PRO593 + [2 × 40 kDa PEG] was retained longer in the vitreous than the parental PRO504, with a measured half-life of 8.0 days and 4.8 days, respectively. The slower vitreal clearance observed with the PEG-conjugated products may allow for less frequent dosing, which could enhance patient adherence and lead to improved treatment outcomes.
References
1. Ferreira A, Sagkriotis A, Olson M, Lu J, Makin C, Milnes F. Treatment frequency and dosing interval of ranibizumab and aflibercept for neovascular age-related macular degeneration in routine clinical practice in the USA. PLoS ONE 2015; 10, e0133968. Crossref
2. Ehlken C, Helms M, Böhringer D, Agostini HT, Stahl A. Association of treatment adherence with real-life VA outcomes in AMD, DME, and BRVO patients. Clin. Ophthalmol. 2018; 12, 13–20. Crossref
3. Shatz W, Hass PE, Mathieu M, et al. Contribution of antibody hydrodynamic size to vitreal clearance revealed through rabbit studies using a species-matched fab. Mol. Pharm. 2016; 13, 2996–3003. Crossref
4. Shatz W, Hass PE, Peer N, et al. Identification and characterization of an octameric PEG-protein conjugate system for intravitreal long-acting delivery to the back of the eye. PLoS ONE 2018; 14, e0218613. Crossref
5. Sharma A, Kumar N, Kuppermann BD, Bandello F. Abicipar pegol: the non-monoclonal antibody anti-VEGF. Eye 2020; 34, 797–801. Crossref
6. Vollmar BS, Fei M, Liang WC, et al. PEGylation of anti-MerTK antibody modulates ocular biodistribution. Bioconjug. Chem. 2022; 33, 1837–1851. Crossref
7. Famili A, Crowell SR, Loyet KM, et al. Hyaluronic acid-antibody fragment bioconjugates for extended ocular pharmacokinetics. Bioconjug. Chem. 2019; 30, 2782–2789. Crossref
8. Chandrasekaran PR, Madanagopalan VG. KSI-301: antibody biopolymer conjugate in retinal disorders. Ther. Adv. Ophthalmol. 2021; 13. Crossref
9. Fisusi F, Brandy N, Wu J, Akala EO. Studies on polyethylene glycol-monoclonal antibody conjugates for fabrication of nanoparticles for biomedical applications. J. Nanosci. Nanomed. 2020; 4, 1–9. Crossref
10. Rodwell J, McKearn T. Linker technology: antibody-mediated delivery systems. Nat. Biotechnol. 1985; 3, 889–894. Crossref
11. Jao SC, Ospina SME, Berdis AJ, Starke DW, Post CB, Mieyal JJ. Computational and mutational analysis of human glutaredoxin (thioltransferase): probing the molecular basis of the low pKa of cysteine 22 and its role in catalysis. Biochemistry 2006; 45, 4785–4796. Crossref
12. Junutula JR, Raab H, Clark S, et al. Site-specific conjugation of a cytotoxic drug to an antibody improves the therapeutic index. Nat. Biotechnol. 2008; 26, 925–932. Crossref
13. Kang JC, Sun W, Khare P, et al. Engineering a HER2-specific antibody–drug conjugate to increase lysosomal delivery and therapeutic efficacy. Nat. Biotechnol. 2019; 37, 523–526. Crossref
14. Park SJ, Choi Y, Na YM, et al. Intraocular pharmacokinetics of intravitreal aflibercept (Eylea) in a rabbit model. Invest. Ophthalmol. Vis. Sci. 2016; 57(6), 2612–2617. Crossref
Affiliations
Stacy L Capehart, Joshua D Slocum, Tobin E Brown, Alexander D Jackson, Samantha R Summers, Peter CS Woodham, Alexei Kazantsev, Marion Weir, and Eric S Furfine, Mosaic Biosciences, Boulder, CO, USA
Sangyuel Han, INGENIA Therapeutics, Cambridge, MA, USA
Authorship & conflict of interest
Contributions: The named authors take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Acknowledgements: None.
Disclosure and potential conflicts of interest: The authors are all current or former employees, and stock holders of Mosaic Biosciences.
Funding declaration: The authors received no financial support for the research, authorship and/or publication of this article.
Article & copyright information
Copyright: Published by Bioconjugation Insights under Creative Commons License Deed CC BY NC ND 4.0 which allows anyone to copy, distribute, and transmit the article provided it is properly attributed in the manner specified below. No commercial use without permission.
Attribution: Copyright © 2025 Mosaic Biosciences. Published by Bioconjugation Insights under Creative Commons License Deed CC BY NC ND 4.0.
Article source: Invited; externally peer reviewed.
Submitted for peer review: Aug 29, 2025.
Revised manuscript received: Oct 28, 2025.
Publication date: Nov 12, 2025.

