Next-generation conjugates: small peptides to transform targeted cancer therapy
Bioconjugation Insights 2025; 1(1), 9–14
DOI: 10.18609/bci.2025.002
Lauren Coyle, Launch Commissioning Editor, Bioconjugation Insights, speaks with Michelle Morrow, Chief Scientific Officer, Avacta, about a novel drug delivery platform that utilizes a small peptide with a capping group conjugated to a cytotoxic cancer drug. She discusses the advantages of the platform over traditional ADC therapies including the improved precision and safety of cancer treatment.
Bioconjugation Insights is the only dedicated provider of specialized, high-quality, Open Access digital content tailored to the bioconjugation field. To read more of our Open Access content, please register here.
Can you tell us a bit about your career, specifically your new role as CSO of Avacta?
MM: My career has been dedicated to the discovery and development of innovative cancer therapies. I began with an academic research career, earning a PhD in immunology at Cambridge, followed by postdoctoral research into childhood leukemia. This research deepened my interest in cancer biology and its underlying mechanisms.
During my postdoctoral research on childhood leukemia at Great Ormond Street Hospital, I gained firsthand experience of the impact of cancer on patients and their families. Motivated by a desire to develop new medicines, I transitioned into the pharmaceutical industry which I have been a part of for nearly 18 years.
My industry career began at MedImmune, now part of AstraZeneca, where I worked primarily in immuno-oncology. I contributed to several significant projects, including the invention of Imfinzi, a PD-L1 checkpoint inhibitor. Subsequently, I joined F-star, a biotechnology company based in Cambridge, which provided the opportunity to work in a smaller, more agile environment while gaining insight into business operations. During my tenure, we successfully advanced multiple molecules from research into clinical trials.
I joined Avacta in 2024 and I was drawn to the company for several reasons. First and foremost is the pre|CISION® platform—a differentiated and versatile technology with significant potential, as approximately 90% of solid tumors express its target, presenting an unprecedented market opportunity. Our mission is to extend the therapeutic index of toxic payloads that effectively target cancer. The company is conducting Phase I clinical studies while simultaneously advancing a discovery engine and preclinical pipeline, which I found particularly compelling. Additionally, I am privileged to work alongside a team of passionate scientists dedicated to advancing these projects.
Could you describe the pre|CISION platform in more detail and how it works?
MM: One of the key challenges in oncology is developing drugs that effectively target cancer cells while minimizing toxicity to patients. Many potent cancer therapies come with significant side effects which can hinder treatment efficacy and allow tumors to regrow. Avacta’s pre|CISION technology addresses this challenge by utilizing a small peptide with an engineered capping group that binds in the groove of the fibroblast activation protein (FAP) enzyme, which can be seen in Figure 1. The pre|CISION peptide is then conjugated to a cytotoxic cancer drug.
| Figure 1. |
|---|
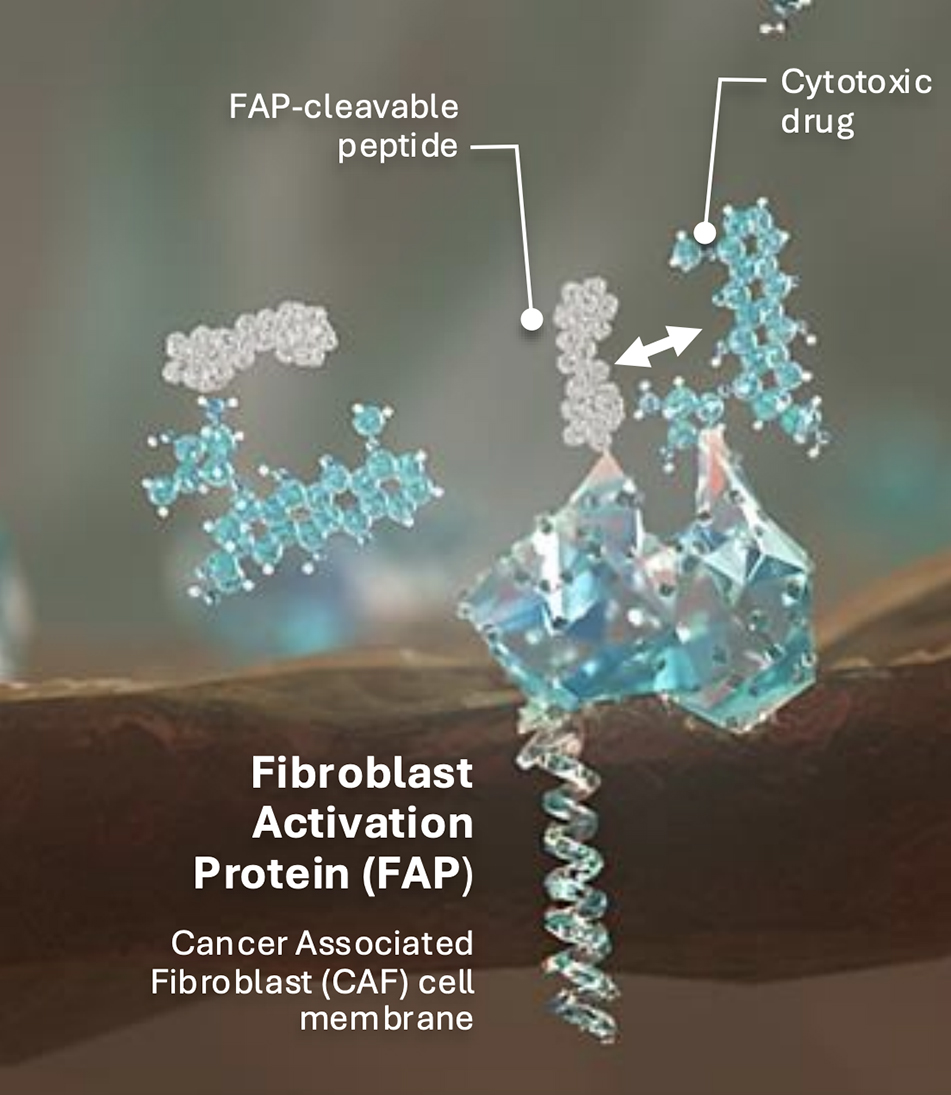 |
In its intact form, the compound remains inactive and cannot enter cells, enabling systemic distribution without causing toxicity. When the conjugated molecule reaches the tumor, the peptide is cleaved by the enzymatic activity of FAP. This cleavage releases the payload from the pre|CISION peptide, allowing it to act specifically within the tumor. This approach enables the administration of higher drug concentrations directly to the tumor, enhancing efficacy while reducing systemic toxicity.
This technology has been applied to various anti-cancer drugs and consistent results have been observed such as potent cancer cell destruction dependent on FAP activity, leading to a broader therapeutic index. These findings have been validated in preclinical mouse models and are now being extended to additional payloads through our clinical and preclinical programs.
Could you tell me what makes FAP strongly attributed to cancer-associated fibrosis (CAF) and why this is so specific?
MM: CAFs are non-cancerous cells within tumors, which are influenced by and can influence the tumor itself, creating the tumor microenvironment (TME). FAP is an enzyme expressed on the surface of fibroblasts, particularly at the interface between the tumor and the stroma, which can be seen in Figure 2. Additionally, it is present in >90% of human cancers. At Avacta, extensive research is being conducted into FAP expression in collaboration with Tempus AI. This collaboration has involved profiling over 160,000 human cancer samples to gain deeper insights into FAP’s role in different cancer types.
| Figure 2. |
|---|
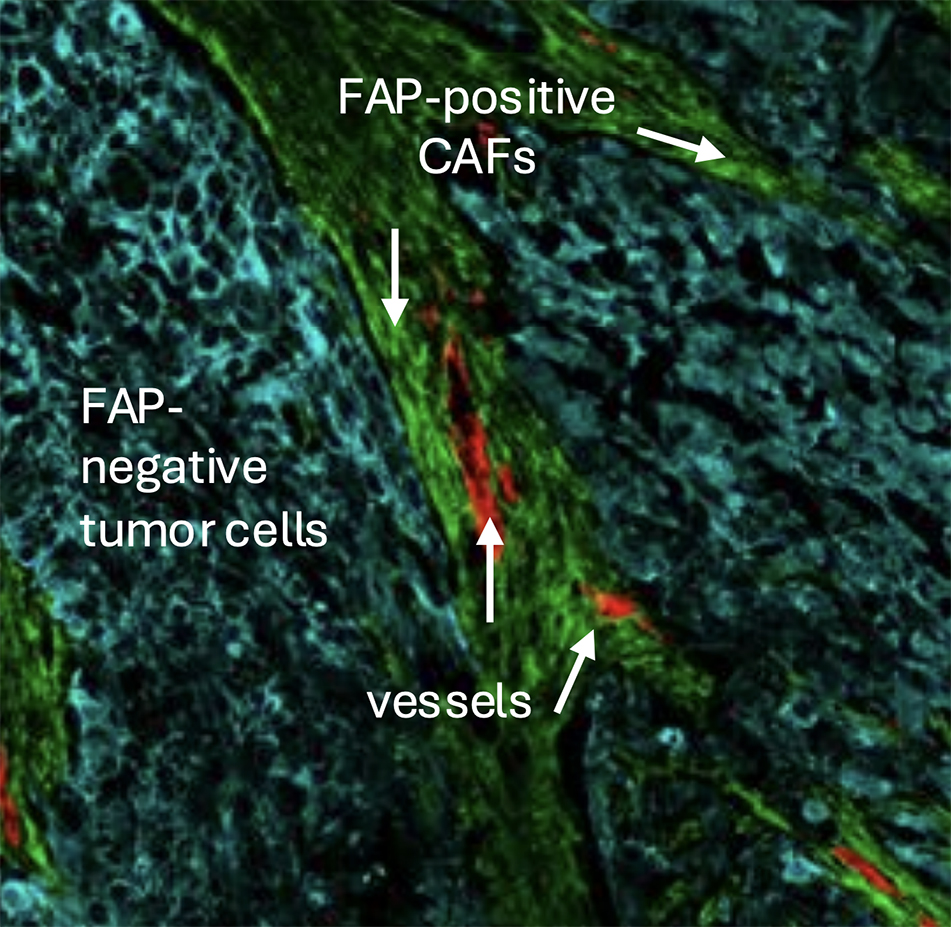 |
The unique advantage of the pre|CISION platform is that it does not only bind to FAP but also leverages its enzymatic activity to release the cytotoxic drug. Avacta is currently the only company utilizing this mechanism to achieve tumor-specific drug activation, making CAFs a crucial component of both tumor biology and the effectiveness of pre|CISION medicines.
Could you tell me about the pharmacokinetic (PK) advantages you have observed with pre|CISION when compared to other drug conjugate platforms?
MM: pre|CISION molecules are small molecule compounds, with the peptide consisting of only two amino acids. Unlike larger peptide-drug conjugates (PDCs), which can be recognized by the immune system, these micro-peptides are not recognized by immune cells, thus minimizing immunogenicity.
Phase I data from FAP-Dox (AVA6000), a FAP-doxorubicin PDC and the first of Avacta’s pre|CISION medicines in clinical trials, has revealed four fundamental changes. First is reduced plasma exposure, ensuring that the payload remains inactive in circulation and minimizing toxicity. Secondly, enhanced tumor exposure allows for more effective drug delivery, with biopsy data from our Phase 1 clinical trial showing a median 100-fold concentration difference between tumors and blood. This is more than what would be expected of an ADC which have reported a three to eight-fold difference. The third and fourth are a reduced volume of distribution and an extended half-life of the released doxorubicin. This lowers active payload exposure in normal tissues and ensures prolonged drug retention in tumors, improving both safety and efficacy through overall reduced cytotoxicity.
The four fundamental changes are based on results seen with FAP-Dox (AVA6000), but this can be applied with other cytotoxic drugs or targeted therapies. Overall, these PK advantages enable a safer, more targeted approach compared to ADCs and traditional chemotherapy.
What have you learned so far from the preclinical and clinical trials about the potential advantages of the pre|CISION platform?
MM: It is exciting to see that these PK differences have also been translated into meaningful clinical outcomes. One of the most promising findings is the improved safety profile of pre|CISION molecules. These have resulted in a reduction of the toxicities typically associated with chemotherapy, providing clinical validation of the platform. Additionally, early clinical data from our Phase I trials have shown encouraging efficacy signals in tumor types known to be sensitive to doxorubicin. As a result, we are now expanding into Phase Ib cohorts to further investigate these effects, particularly in salivary gland cancer and triple-negative breast cancer.
Another exciting development at Avacta is the FAP-Exatecan project. Exatecan (EXd) is a potent cytotoxic agent and a type I topoisomerase inhibitor, a class of molecules that has already been successfully used in ADCs. The preclinical trials for this project have just been completed and showed highly promising results. Since delivering this class of cytotoxic drugs to tumors has proven effective in patients, there is great potential for the FAP-EXd molecule, AVA6103.
In our preclinical mouse tumor models, FAP-EXd (AVA6103) has demonstrated remarkably strong responses, including complete tumor regression at well-tolerated levels. The molecule has been shown to be tolerated at doses up to 75 times higher than conventional EXd alone. This suggests a significantly improved therapeutic window, giving us confidence in its potential clinical benefits. We are now initiating IND-enabling studies for this program and are eager to move it forward, as we believe it could have a transformative impact on patient treatment in the future. We anticipate the Phase 1 trial beginning in the first quarter of 2026
Overall, what are the main differences between PDC and traditional ADCs, and what advantages and/or challenges have you seen so far?
MM: The platform shares common goals with ADCs, aiming to deliver toxic drugs to tumors while minimizing side effects. However, the two differ significantly in their molecular composition. The pre|CISION molecules are small, allowing for better tumor penetration compared to antibodies. This enhanced penetration can potentially lead to higher drug concentrations in tumors than what is achievable with conventional ADCs.
There are some key advantages of pre|CISION PDCs over conventional ADCs. Avacta has the ability to fine-tune the kinetics how the cytotoxic payload is released in a FAP-dependent manner. For instance, with AVA6103, modifications to the technology were introduced by incorporating additional linker groups, which enables the control rate at which FAP cleaves the molecule to achieve a sustained release of EXd in the tumor microenvironment.
pre|CISION molecules also exhibit a bystander effect as the warheads are released in the TME rather than inside individual cancer cells. This means that they can effectively kill neighboring cancer cells unlike conventional ADCs, which rely on antigen expression on tumor cells for direct intracellular delivery. ADCs also face challenges related to resistance, which may be a limitation of their mechanism of action.
Immunogenicity is another major consideration. Antibodies inherently carry the risk of triggering anti-drug antibody responses, which can impact both the efficacy and PD of the drug while causing side effects for patients. With pre|CISION, this is not a concern as it does not involve antibody-based delivery.
Finally, from a manufacturing perspective, the platform benefits from small-molecule synthesis, which is significantly less expensive and time-consuming compared to the complex production processes required for ADCs. This streamlined manufacturing allows for a more rapid transition from research to clinical application. While there are similarities between pre|CISION and ADCs, the differences present clear advantages that we believe make it a highly promising platform.
Finally, what are your overachieving goals for the next year or two?
MM: My primary focus is advancing the FAP-Exatecan program (AVA6103) through IND-enabling studies. We aim to submit the IND later in the year and initiate clinical trials in early 2026, marking a significant milestone for Avacta. Additionally, we are expanding our discovery efforts to explore new payloads that could be optimized using the platform.
We are also refining the technology to enhance its performance further. Looking ahead, we are excited to continue generating clinical data with key data readouts from our Phase 1b cohorts in 2025. It will be an exciting few years for Avacta and we look forward to sharing further progress.
Biography
Michelle Morrow is the Chief Scientific Officer at Avacta Therapeutics, a biotech company focused on developing cancer treatments using its pre|CISION® peptide drug conjugate platform. Previously, Morrow was SVP and Head of InvoX Therapeutics Innovation at InvoX Pharma, after its acquisition of F-star Therapeutics. At F-star, she led teams that advanced several bispecific antibody therapeutics from discovery to clinical trials. Earlier, she held leadership roles at Medimmune (AstraZeneca), where she contributed to the development of Imfinzi® (an approved anti-PD-L1 antibody) and volrustomig (an anti-PD-1/CTLA-4 bispecific antibody). Morrow earned a PhD in Immunology from the University of Cambridge, Cambridge, UK and conducted post-doctoral research on childhood leukemia at the Institute of Child Health in London.
Affiliation
Michelle Morrow PhD, Chief Scientific Officer, Avacta Therapeutics, London, UK
Authorship & Conflict of Interest
Contributions: The named author takes responsibility for the integrity of the work as a whole, and has given his approval for this version to be published.
Acknowledgements: None.
Disclosure and potential conflicts of interest: The author has no conflicts of interest.
Funding declaration: The author received no financial support for the research, authorship and/or publication of this article.
Article & Copyright Information
Copyright: Published by Bioconjugation Insights under Creative Commons License Deed CC BY NC ND 4.0 which allows anyone to copy, distribute, and transmit the article provided it is properly attributed in the manner specified below. No commercial use without permission.
Attribution: Copyright © 2025 Michelle Morrow. Published by Bioconjugation Insights under Creative Commons License Deed CC BY NC ND 4.0.
Article source: Invited.
Revised manuscript received: Mar 21, 2025.
Publication date: Apr 25, 2025.


